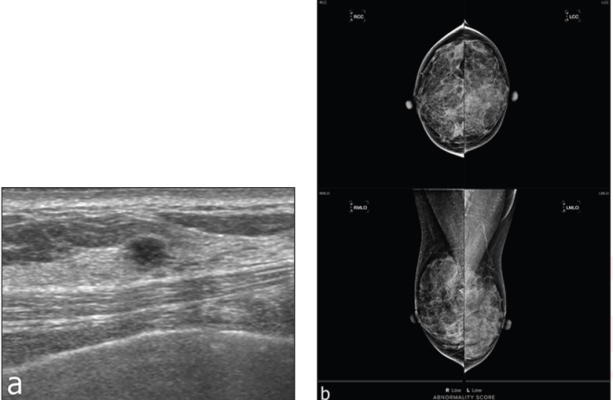
57-YO woman with dense breasts—screening mammography (not shown) assessed BI-RADS 2. a. Supplementary screening breast ultrasound performed on same day as mammogram shows 4-mm round hypoechoic mass with angular margins in right upper medial breast. b. Screenshot of output of AI tool. Tool assigned abnormality score of “low” in each breast. Ultrasound-guided core biopsy and surgery revealed invasive ductal carcinoma, not otherwise specified (Luminal A, histologic grade I). Case represents false-negative AI result.
July 28, 2023 — Findings from an accepted manuscript published in the American Journal of Roentgenology (AJR) suggest that for patients with dense breasts undergoing screening in the incidence setting, a commercial AI tool did not provide additional benefit to mammography with supplementary ultrasound (US).
“Mammography with supplementary ultrasound showed higher accuracy, higher specificity, and lower recall rate in comparison to mammography with AI, as well as in comparison to mammography with both US and AI,” wrote corresponding author Hee Jung Moon, MD, PhD, from the department of radiology at South Korea’s Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine.
This AJR accepted manuscript included 1,325 women (mean age, 53 years) with dense breasts who underwent both screening mammography and supplementary breast US within a 1-month interval from January 2017 to December 2017; prior mammogram and US were available to compare in 91.2% and 91.8%, respectively. Fifteen radiologists (5 staff and 10 fellows) interpreted mammography and US examinations, and clinical reports were used for Moon et al.’s analysis. A commercially available AI algorithm (Lunit INSIGHT, v 1.1.0.0, Seoul, Korea) was used to retrospectively evaluate mammographic examinations for cancer presence. Then, screening performances were compared among mammography, AI, US, and test combinations, using generalized estimating equations. At least 24 months of imaging stability was required for a benign diagnosis.
Ultimately, mammography with AI, mammography with US, and mammography with both ultrasound and AI showed recall rate of 14.9, 11.7, and 21.4 (all p < .05); sensitivity of 83.3%, 100.0%, and 100.0% (all p > .05); specificity of 85.8%, 89.1%, and 79.4% (all p < .05); and accuracy of 85.7%, 89.2%, and 79.5% (all p < .05).
For more information: www.arrs.org


 February 18, 2026
February 18, 2026 









