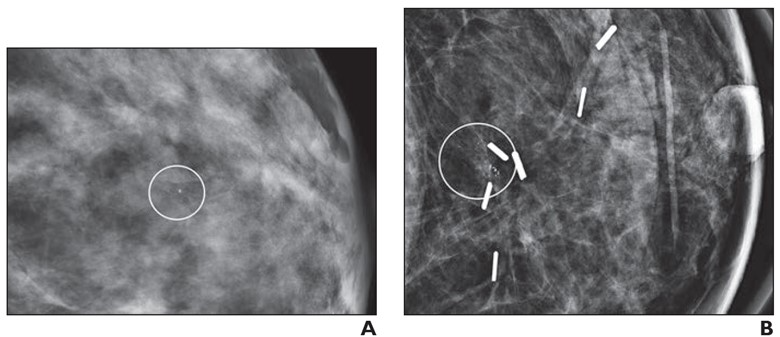
48-year-old woman with recurrent ductal carcinoma in situ (DCIS) in left breast. A. Mammographic magnification view shows grouped calcifications in left breast (circle). Stereotactic biopsy yielded grade 2 DCIS. Patient underwent lumpectomy with tumor-negative surgical margins and radiation therapy. B. Mammographic magnification view obtained 4 years after diagnosis shows new calcifications at surgical site (circle). Stereotactic biopsy was performed, which revealed recurrent grade 2 DCIS. Patient then underwent mastectomy.
December 26, 2023 — Results published in the American Journal of Roentgenology (AJR) support mammographic surveillance protocol for patients with a previous breast cancer diagnosis of returning immediately to digital breast tomosynthesis (DBT) screening.
“Although the abnormal interpretation rate (AIR) was higher during the year after diagnosis compared to subsequent years, the AIR remained acceptably low (< 10%) in all years,” concluded lead investigator Dan Do, BA, from Massachusetts General Hospital in Boston.
This AJR accepted manuscript included 8,090 patients (mean age, 65 years) with a prior breast cancer diagnosis who underwent 30,812 screening DBT examinations during Do et al.’s study period. The cancer detection rate (CDR) was 8.6 per 1,000 examinations, AIR was 5.7%, PPV 1 was 15.1%, sensitivity was 80.3%, specificity was 95.1%, and false-negative rate was 2.1%.
Ultimately, CDR showed a significant independent positive association with years since breast cancer diagnosis (adjusted OR 1.03, 95% CI 1.01-1.05, p<.001). Meanwhile, AIR evidenced significant independent negative association with years since breast cancer diagnosis (adjusted OR 0.99, 95% CI 0.98-1.00, p=.01)—being highest less than or equal to 1 year after diagnosis (7.5%) and lowest greater than 5, but less than 6 years postdiagnosis (5.0%).
For more information: www.arrs.org


 February 18, 2026
February 18, 2026 









