May 8, 2023 — RapidAI, the global leader in using AI to combat life-threatening vascular and neurovascular conditions and support patient workflow, announced today that its combined hemorrhagic solution — Rapid ICH and Rapid Hyperdensity — has been selected as the “Best New Imaging Solution” in the 7th annual MedTech Breakthrough Awards, an independent market intelligence organization that recognizes the top companies, technologies, and products in the global health and medical technology market. This year’s program attracted more than 4,000 nominations from over 17 different countries.
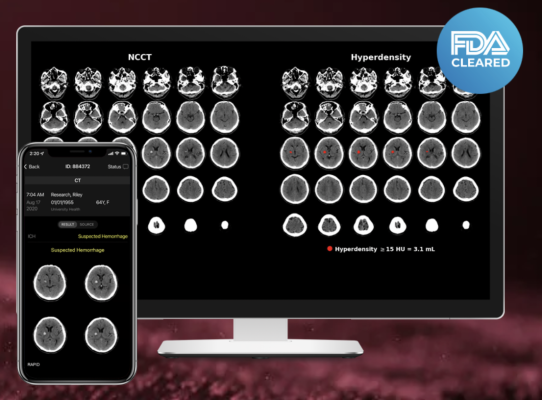
RapidAI was chosen for the solution’s meaningful impact on the treatment of acute hemorrhagic stroke and head trauma care in hospitals around the world. When it comes to brain hemorrhage, speed and accuracy in diagnosis are essential. Rapid ICH and Rapid Hyperdensity use AI to quickly assess non-contrast CT (NCCT) scans for suspected hemorrhage and automatically calculate the hyperdense volume output in minutes. For hospitals and mobile stroke units on the front lines of patient assessment, this additional contextual data is critical to helping them make faster and more informed triage and transfer decisions.
“Rapid ICH and Rapid Hyperdensity represent breakthrough solutions to support physicians in managing care for patients with brain hemorrhages – a condition that impacts tens of thousands of people every year,” said James Johnson, managing director, MedTech Breakthrough. “The RapidAI hemorrhagic solution gives physicians an incredibly high degree of confidence to make critical decisions about patient care. We’re proud to award RapidAI with the ‘Best New Imaging Solution’ award.”
RapidAI’s industry-leading suite of stroke solutions improve diagnostic accuracy, allow for expedited decision-making, and streamline care, ultimately leading to better patient outcomes. In the last year alone, the company announced 3 other FDA clearances – Rapid PE Triage & Notification, Rapid RV/LV, and most recently Rapid NCCT Stroke – and was recognized by Frost & Sullivan with the 2022 Global Technology Innovation Leadership Award. RapidAI is also fast-approaching an important milestone of 10 million patient scans – a 50% increase in the past year, demonstrating the immense impact of the technology on patient care.
“We are thrilled to receive the MedTech Breakthrough Award in recognition of our efforts to revolutionize care for hemorrhagic stroke patients,” said Karim Karti, CEO of RapidAI. “We’ve accomplished so much in the past year – including several FDA 510(k) clearances, crossing the 10 million patient scans milestone, and several awards – but this is only the beginning. I am incredibly proud of the impact the team has made and look forward to our continued game-changing innovation in the treatment of vascular and neurovascular conditions.”
For more information? https://www.rapidai.com/


 February 13, 2026
February 13, 2026 









