May 21, 2020 — RADLogics announced that its AI-powered medical imaging applications designed to assist in the detection and quantification of findings associated with COVID-19 will now be available on the Nuance AI Marketplace. The Nuance AI Marketplace is the first and largest portal with one-stop access to a wide range of AI diagnostic models from within the industry’s most widely used radiology reporting platform.
Following the announcement of global deployments of RADLogics’ AI-powered devices, the availability of RADLogics’ applications on the Nuance AI Marketplace include patient triage and disease extent measurements, using imaging findings on computed tomography (CT) and X-ray scans. Access to these applications will help meet the growing demand in the U.S. for these solutions that have the capacity to process 1 million CT and 10 million X-rays studies per day through the RADLogics’ cloud-based platform.
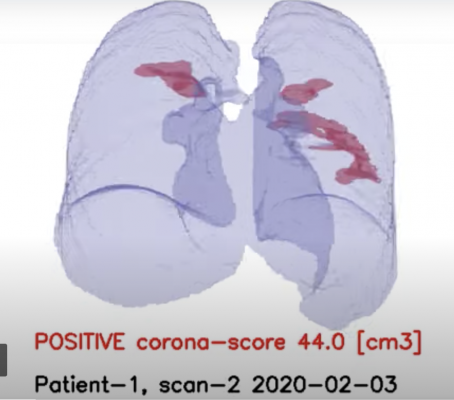
RADLogics’ AI-powered software includes algorithms that not only detect abnormalities on chest CTs and X-rays, but also provide automatic triage alerts to the radiologist to help ensure potential findings are reviewed in a timely matter. In addition, the AI-Powered devices provide quantitative analysis of the CT and X-ray images for patients with suspected COVID-19 disease including a score that can help monitor findings over time. In response to the COVID-19 pandemic, RADLogics has dedicated the company’s resources to modify, adapt, and deploy its algorithms to detect lung abnormalities that are compatible with COVID-19 in appropriate clinical settings.
The Nuance AI Marketplace functions like an app store dedicated to radiology. It gives algorithm developers consolidated, at-scale access to users of Nuance PowerScribe™, the radiology reporting system trusted by approximately 80 percent of U.S. radiologists across more than 7,000 connected healthcare facilities. Radiologists can discover, test, and use AI models from within their familiar PowerScribe reporting and worklist workflows to increase reporting efficiency and quality, and to help care teams improve performance and reduce healthcare costs. A built-in feedback channel lets users share real-world results with developers for AI model refinement and post-market surveillance. Hospital systems benefit with simplified purchasing and metrics showing model usage, costs, and performance.
“We are committed to meet the growing demand for our AI-Powered medical imaging analysis solutions during the pandemic by making our applications available to U.S. clinicians and radiology teams through the revolutionary Nuance AI Marketplace,” said Moshe Becker, CEO and Co-Founder of RADLogics. “We are dedicated to supporting the fight against COVID-19, and Nuance’s cloud-based marketplace will facilitate the deployment of our applications to support healthcare systems and providers throughout the U.S. as they continue to treat symptomatic patients.”
In accordance with recent FDA guidance for imaging systems and software to address the COVID-19 public health emergency, RADLogics is committed to making its CT and X-ray solutions available to hospitals and healthcare systems throughout the U.S. for patient triage and management. Designed for easy integration and installation both on-premise and via the cloud – RADLogics’ algorithms are supported by the company’s patented workflow software platform that enables rapid deployment at multiple hospitals, and seamless integration with existing workflows.
For more information: www.radlogics.com


 February 13, 2026
February 13, 2026 









