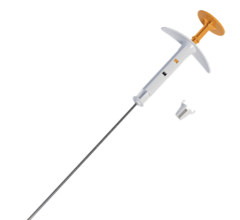
April 23, 2009 - A new study shows that the SAVI applicator, a small, expandable device inserted inside the breast to deliver partial breast irradiation, carries a low infection risk, a potential complication of such devices.
The research, led by radiation oncologists and surgeons at the Moores UCSD Cancer Center and Fort Myers, Florida-based 21st Century Oncology, also indicates that other complications – such as seromas, pockets of fluid that build with the use of internal radiation devices – are unlikely to occur.
That’s good news for those women with early-stage breast cancer who opt to have such devices inserted for their radiation therapy after breast-sparing lumpectomy surgery, said Catheryn Yashar, M.D., associate professor of radiation oncology at the UC San Diego School of Medicine and chief of breast and gynecological radiation services at the Moores UCSD Cancer Center. Their use is increasing, she added, noting that the Moores UCSD Cancer Center was one of the first medical facilities in the country to offer SAVI.
SAVI, which consists of flexible catheters through which radiation is given, provides customized radiation therapy and minimizes exposure to healthy tissue after a woman has undergone a lumpectomy to remove a cancerous tumor. Radiation specialists sometimes decide to give women internal radiation – a process called brachytherapy – with the goal of giving concentrated doses of radiation to areas of concern while avoiding healthy tissue.
In the study, researchers examined one-year follow-up data on 63 patients treated with the Food and Drug Administration-approved SAVI device. They found an infection rate that is less than half of the published rates associated with balloon brachytherapy methods, and rated overall cosmetic outcomes with SAVI as “excellent.” The results will be presented at the American Society of Breast Surgeon’s annual meeting in San Diego, April 24, 2009.
In addition, physicians were able to use the device’s many catheters to customize the radiation dose based on the woman’s needs, greatly minimizing radiation to the heart, lungs, ribs and skin, likely resulting in fewer complications, Yashar said. To date, there have been no recurrences or formation of persistent seromas.
“With a full year of follow-up, our research confirms previous findings that this device is safe and effective for radiation delivery, especially compared to other brachytherapy methods,” said Yashar. “Without the ability to customize the dose, other devices can lead to complications, like persistent seroma and skin burns. This applicator was created to overcome these problems, and our research shows it has been successful.”
Breast brachytherapy is a form of Accelerated Partial Breast Irradiation (APBI). Lasting just five days, APBI offers a shorter course of radiation compared to the six weeks required with traditional whole breast irradiation.
“SAVI has the most flexible dose modulation for single-entry APBI applicators and can sculpt the radiation dose to the size and shape of the tumor cavity and the patient’s anatomy, even when only one to two millimeters from normal tissues,” Yashar said.
Without the technical limitations of other methods such as balloon brachytherapy, SAVI substantially increases the number of women who qualify for the benefits of APBI.
Other authors of the poster being presented at ASBS are Daniel Scanderbeg, Anne Wallace, Sarah Blair and Patrick Barna, UC San Diego; and Constantine Mantz, 21st Century Oncology. The SAVI breast brachytherapy applicator is made by Cianna Medical, Inc.
The Moores UCSD Cancer Center is one of the nation’s 41 National Cancer Institute-designated Comprehensive Cancer Centers, combining research, clinical care and community outreach to advance the prevention, treatment and cure of cancer.
For more information: www.cancer.ucsd.edu and www.ciannamedical.com


 October 01, 2023
October 01, 2023