October 21, 2020 — Quantib, a market leader in artificial intelligence solutions for radiology, today announced that it received FDA clearance for its prostate solution. The solution is a zero-footprint software providing a comprehensive and intuitive workflow for enhanced assessments and is paired with fast processing and image registration enabling real-time workflows.
The release of Quantib’s prostate solution comes after PROMISS and Precision trials indicated the added value of incorporating MRIs in the prostate cancer workflow. Subsequently, guideline changes from the ACR (American College of Radiology) advising that MRIs become standard prior to biopsies adds further to the importance of prostate diagnosis within radiology. Yet with the high level of expertise required for prostate diagnoses, there aren’t enough radiologists to meet growing demands on their own.
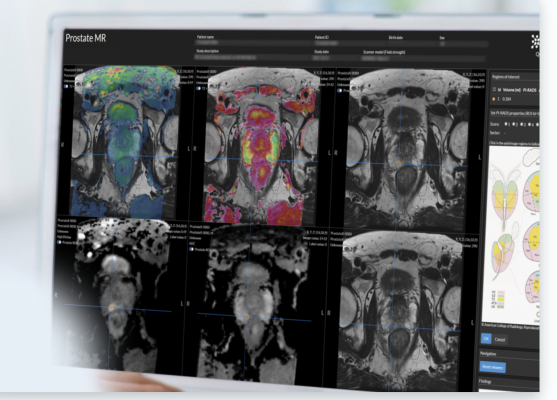
Quantib’s AI prostate solution comes with a suite of tools to improve diagnoses, including an image-based calculation of PSA density that is automatically incorporated into the final radiology report and a bi-parametric heat map representation that highlights suspicious areas to support the radiologist with easy and fast detection of lesions. The solution enables an intuitive and agile process for the assessment of ROIs and the determination of the PI-RADs score, while simultaneously standardizing reporting to facilitate easy and comprehensive communication of results.
Following its FDA clearance, Quantib plans to deploy in 10 facilities this year and will open a waitlist for 2021, with rapid adoption and expanded accessibility a priority. In addition, Quantib is developing enhanced features to be made available over the coming months.
“Quantib’s FDA clearance is a game-changer for American radiologists who will, for the first time, have the powerful AI solutions they’ve been eager to implement for one of their most common disease areas,” said Arthur Post Uiterweer, CEO of Quantib. “Both the instant impact and potential of this solution are significant for radiologists and patients alike. We aim to magnify that impact by focusing on widespread adoption and routine upgrades. ”
“As Europe’s largest treatment center for prostate cancer, we see big challenges in the diagnostic pathway that AI should be addressing.” said Detlef Loppow, M.D., CEO of Martini Klinik, Hamburg, Germany. “On a weekly basis we are working with Quantib’s brilliant engineers to create a truly groundbreaking pipeline of innovations. I’m thrilled to see the first release entering the market now for clinical use.”
For more information: www.quantib.com


 February 13, 2026
February 13, 2026 









