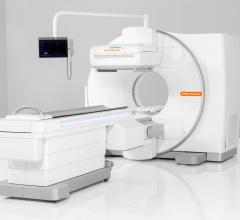
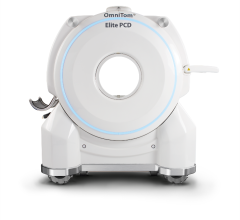
inSPira HD is a portable high resolution SPECT system.
September 1, 20009 - The U.S. Food and Drug Administration (FDA) granted 510(k) clearance for a new portable high-resolution single photo emission computed tomography (SPECT) system, inSPira HD, NeuroLogica Corp. announced today at the XIV World Congress of Neurological Surgery in Boston, Mass. The inSPira HD is a battery powered, high resolution, portable SPECT designed primarily for brain imaging. Its reportedly provides high quality SPECT images at a clinic, ICU, OR and emergency department. inSPira HD is said to be capable of imaging any radioisotope energies between 80 - 200 keV for clinical applications such as epilepsy, Parkinson's, stroke and Alzheimer's dementia, said the manufacturer. Traditional systems whose basic principles have been around since the 1960's continue to suffer from poor spatial and contrast resolution. This has led to the term "fuzzy" imaging with 5-10mm spatial resolution in any one axis. The inSPira HD presents an entirely new method of SPECT detection, acquisition and reconstruction, which has been designed to approach 3-mm spatial resolution. For more information: www.neurologica.com


 February 15, 2024
February 15, 2024