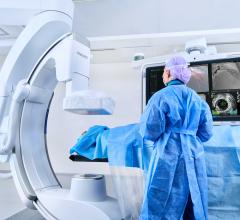
September 13, 2017 — Philips announced its presence at the Vascular Interventional Advances (VIVA 17) Annual Conference in Las Vegas from Sept. 11 – 14, 2017, applying advanced technologies and deep clinical insights to address peripheral vascular needs. With the addition of its recent acquisition, Spectranetics, Philips will for the first time highlight its expanded portfolio of interventional X-ray systems, advanced catheters for measurement and therapy, clinical informatics and services, including its Philips Azurion image-guided therapy platform, intravascular ultrasound (IVUS), Phoenix atherectomy system and Spectranetics' Stellarex drug-coated balloon.
More than 8 million people in the U.S. alone are affected by peripheral arterial disease (PAD) [1]. Many people do not experience symptoms and are not aware of even having the disease. In addition to having limited blood flow to the leg, patients are at increased risk for heart attack or stroke. Fortunately, PAD can be accessed and addressed through innovative solutions using minimally invasive procedures like atherectomy and cardiac imaging technology that may offer more accurate and reliable treatment options that can reduce recovery time for patients.
On Wednesday, Sept. 13, as part of the VIVA 17 Late-Breaking Clinical Trials program, Marianne Brodmann M.D., of the Medical University of Graz in Austria will present two-year data from the ILLUMENATE European randomized clinical trial (EU RCT) that evaluates how Spectranetics' Stellarex drug-coated balloon restores and maintains blood flow to arteries in patients with PAD. Stellarex received U.S. Food and Drug Administration (FDA) approval in July 2017. The ILLUMENATE EU RCT analyzes patency in the segment of diseased artery treated with Stellarex in over 300 patients from a challenging patient population.
The combination of Spectranetics' product range and Philips' portfolio of interventional imaging systems, devices, software and services offer innovative solutions for peripheral vascular therapy, according to the company. At this year's VIVA conference, Philips and Spectranetics will showcase technology including:
- Stellarex, the only commercially available drug-coated balloon with two reported randomized controlled trials. It has demonstrated durability with consistently high patency rates in a wide range of patients, according to Philips;
- Azurion is an image-guided therapy platform that enables clinicians to easily and confidently perform a range of routine and complex procedures, helping them to optimize interventional lab performance;
- The Pioneer Plus catheter, a re-entry device with intravascular ultrasound (IVUS), a catheter-based imaging technology that allows physicians to visualize diseased vessels from inside the artery, to facilitate identification of true lumen location;
- The Phoenix atherectomy system, which combines the benefits of existing atherectomy systems and delivers a hybrid [2] atherectomy option to help physicians tailor the treatment approach for each patient; and
- SymphonySuite is Philips' comprehensive program that includes a robust set of tools to support efforts in opening, growing and maintaining office-based lab solutions.
For more information: www.usa.philips.com/healthcare
References
1. National Institutes of Health, Facts About Peripheral Arterial Disease (P.A.D.), NHLBI.NIH.gov, September 2017
2. Directional cutting ability only available with Phoenix 2.4mm deflecting catheter

 March 05, 2026
March 05, 2026