October 26, 2018 — Philips announced what it calls its ultimate ultrasound solution for breast assessment, available with the Philips Epiq and Affiniti ultrasound systems, and the combination of the eL18-4 transducer and anatomical intelligence. This all-in-one solution brings together high-quality imaging with complementary clinical tools tailored for breast screenings. With the new breast solution, clinicians can efficiently assess, monitor and treat breast diseases, increasing diagnostic confidence and helping to improve patient care.
The Philips ultimate ultrasound solution for breast assessment is CE marked and has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
Many clinicians depend on ultrasound to enhance detection of breast cancers, especially in women with dense breast tissue, which is unrelated to weight or breast size. According to a report published by the Journal of the US National Cancer Institute, an estimated 43.3 percent of women between the ages of 40 and 74 years old have extremely dense breast tissue [1].
With X-ray mammography, dense breast tissue can sometimes mask small cancerous lesions. Clinicians often rely on ultrasound as a follow-up diagnostic test in cases where lesions are suspected. Developed in partnership with clinicians around the world, the Philips ultimate ultrasound solution for breast assessment empowers clinicians to detect breast lesions with confidence and allows them to do so in an intuitive, efficient way. Together, this solution enhances the patient experience by reducing total exam time, eliminating the need for inconvenient room or equipment changes, and avoiding multiple appointments which can result in additional anxiety for patients awaiting diagnosis.
“The best way to increase the survival rate from breast cancer is to detect it early,” said Marcela Böhm-Vélez, M.D., Weinstein Imaging Associates, Pittsburgh. “For women with dense breasts, ultrasound can be very helpful in detecting masses not easily seen on the mammogram. I have been impressed by Philips’ continued investment and innovation in developing breast assessment solutions, so that I can provide optimal care for my patients.”
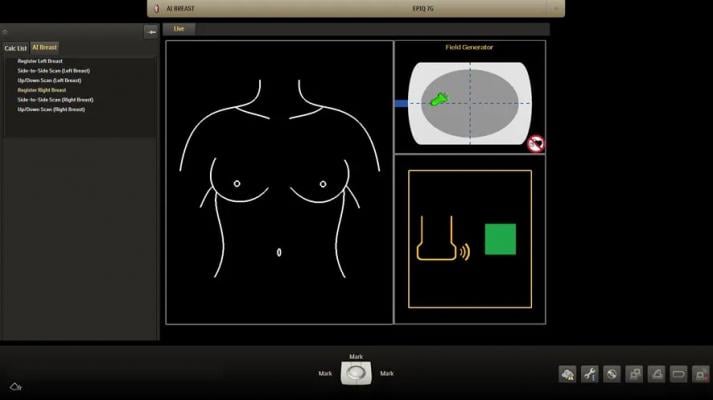
Philips’ integrated breast solution combines four features that work together for comprehensive and confident screening and diagnosis. The PureWave eL18-4 ultra-broadband linear array transducer is a single transducer that delivers high-quality imaging for patients, including those who may be more technically challenging to image. PureWave crystal technology delivers fine-elevation focusing to provide exceptional detail resolution and tissue uniformity, as well as extended depth of field performance.
Anatomical intelligence for breast enhances reproducibility and streamlines workflow while preserving image quality during the breast exam. The automation and intelligence provides visual mapping and annotation of screened anatomy, with minimal user interaction, to give clinicians confidence while documenting full coverage of the breast during the acquisition phase.
Full solution elastography ElastQ Imaging shear wave and strain elastography allows clinicians to rapidly assess a wide array of breast lesions with elevated diagnostic confidence. In combining both wave methods, ElastQ Imaging reveals more definitive information on tissue stiffness in the breast.
A new precision biopsy allows physicians to enhance the patient experience by performing more targeted biopsies. Reduced blind zones and enhanced needle reflections elevate clinical during interventional procedures.
For more information: www.usa.philips.com/healthcare
Reference
[1] Brian L. Sprague, Ronald E. Gangnon, Veronica Burt, Amy Trentham-Dietz, John M. Hampton, Robert D. Wellman, Karla Kerlikowske, Diana L. Miglioretti; Prevalence of Mammographically Dense Breasts in the United States, JNCI: Journal of the National Cancer Institute, Volume 106, Issue 10, 1 October 2014, dju255, https://doi.org/10.1093/jnci/dju255


 February 18, 2026
February 18, 2026 









