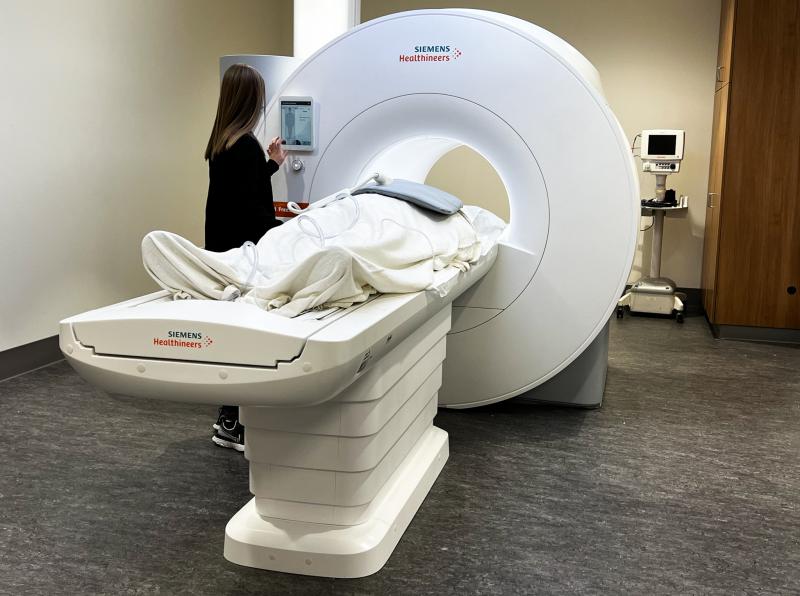
December 17, 2021 — MRI is a powerful medical tool that provides detailed images of everything from bones and joints to the brain and spinal cord, but millions of patients can’t benefit from the improved care it provides. Implanted devices like defibrillators and pacemakers interfere with the MRI’s magnetic signal, while obese and claustrophobic patients are often unable to get into the small opening on a traditional MRI machine. Now, The Ohio State University Wexner Medical Center is the first in the nation to install a reimagined MRI machine built by Siemens in collaboration with Ohio State researchers that was recently approved for use in patients by the FDA. It has a much lower magnetic field and a larger patient opening, removing barriers to this potentially life-saving technology.
“My colleagues and I developed new techniques to boost the signal-to-noise ratio in MRI machines, which made us think that we could use this technology to create a machine with a lower magnetic field strength and still get high quality images out,” said Orlando Simonetti, professor of internal medicine and radiology at The Ohio State University College of Medicine.
In some instances, the low-power machine actually provides better imaging than a traditional MRI, especially of the heart and lungs. It’s an important advancement for patients with conditions like heart failure, cystic fibrosis, pulmonary hypertension and even COVID-19, who must otherwise undergo imaging that uses harmful radiation such as X-rays or CT scans.
“Because of the air in the lungs, it cancels out the signal at higher field strength. But at lower field strength, there’s potential that we can see lung tissue more clearly with MRI,” Simonetti said.
Ohio State has partnered with Nationwide Children’s Hospital to study the use of this new MRI technology for kids with congenital heart disease, who must undergo repeated heart catheterizations throughout their lives. Until now, the procedure required X-ray imaging to guide small metal wires. But the new MRI machine does not interact with the wires, making the procedure safer and preventing these children from being exposed to radiation each time it’s performed.
For more information: www.osu.edu
Watch VIDEO: New MRI Safer to Scan Patients With Implantable Devices


 February 09, 2026
February 09, 2026 









