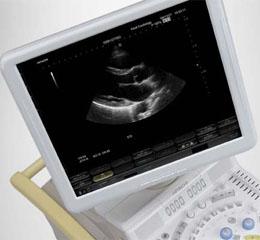
August 24, 2010 - An ultrasound scanner that packages imaging electronics with ergonomics into a compact, user-friendly system is now available in the U.S. market and has been available in Europe and Japan for more than a year.
The HI VISION Preirus ultrasound scanner from Hitachi Medical Systems America Inc. is the first ultrasound system to use Cell Processor technology and “Smart Touch” operation featuring a touch-screen integrated with the LCD display for increased scanning efficiency.
Preirus is complemented by a wide selection of transducers. Each probe is engineered with application-specific crystal array characteristics and architecture, from Hitachi’s V53W transvaginal probe with its slender design and 200° field of view to its 4D volumetric imaging probes, both linear and curved, or its laparoscopic probe with a thin insertion shaft enabling the use of a 10 mm trochar.
The Preirus also supports advanced imaging with optional features of real-time tissue elastography, which applies the fundamental concept of palpation to ultrasound imaging, and real-time virtual sonography, which correlates MRI, CT or ultrasound volumes to a live ultrasound exam and displays them both side-by-side in real-time during scanning.
For more information: www.hitachimed.com


 February 05, 2026
February 05, 2026 









