Siemens Healthcare said its new molecular CT is designed to provide a premium-imaging platform and is a practical solution for today’s shrinking healthcare budget.
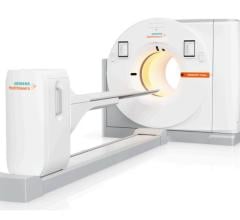
The new technology, Biograph mCT, combines a CT with molecular capabilities, a PET scanner with unlimited capabilities in CT and a high performance integrated imager that can be used to achieve true collaboration and cooperation between diagnostic imaging specialties, according to the company.
Biograph mCT is designed to obtain functional, anatomical and molecular information from one, noninvasive diagnostic exam, and aims to adapt to virtually any patient and any clinical need with higher resolution, contrast and speed. The clinician has the option of using CT in conjunction with the high-definition PET technology or as a standalone CT. Depending on the case, molecular contrast can be used for additional metabolic information. Biograph mCT is reportedly the only imaging device to offer whole-body PET-CT scanning in just five minutes.
With a small footprint, large bore, a short tunnel and an extra-wide, 500-pound-capacity bed (227 kg), Biograph mCT is designed to accommodate all kinds of patients -- including pediatric, bariatric and geriatric -- comfortably and easily. Biograph mCT’s large 78 cm bore can also help to alleviate patients’ sense of claustrophobia and allows better positioning of RTP devices, such as breast boards, and makes scanning of obese patients easier.
Biograph mCT adapts to virtually all patient types and clinical needs. Its sotropic resolution in CT brings out the finest anatomical details in every scan -- routine and advanced -- limiting motion artifacts. It is available in detector configurations of 40-, 64-, and 128-slices, a first in integrated scanners. Biograph mCT’s HD-PET technology delivers detailed images with up to four times as much contrast. It is reportedly the only PET technology that offers 2 mm uniform resolution throughout the field of view (FOV).
Biograph mCT also addresses two of the foremost concerns related to scanning: scan time and radiation dose. With its ultra-low dose capability and super-fast PET acquisition time, scanning can be less stressful for the clinician and the patient, according to Siemens.


 February 27, 2026
February 27, 2026