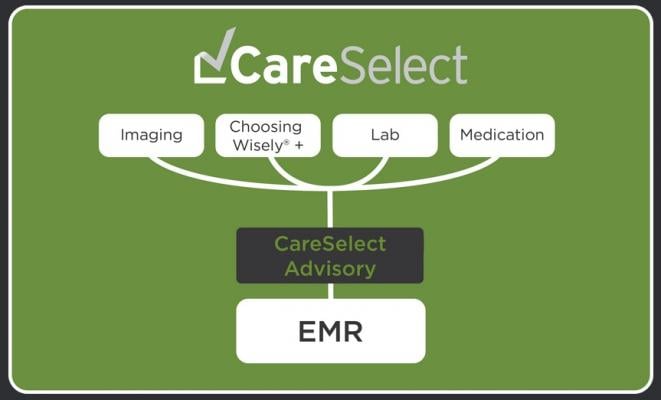
December 6, 2016 — National Decision Support Company (NDSC) recently announced expanded CareSelect solutions that cover a wide variety of care settings and healthcare services including the ABIM Foundation's Choosing Wisely Campaign.
NDSC's CareSelect helps to accurately render clinical guidelines for electronic medical record (EMR) delivery, leveraging existing clinical documentation and creating a seamless user experience within the EMR workflow. The expanded solution now includes clinical and business logic covering medication, lab and blood management, as well as a complete set of advisories based on the Choosing Wisely Campaign.
The CareSelect platform exchanges data with the EMR in real time to perform clinical calculations against evidence-based guidelines and pathways. This enables the caregiver to choose alternate tests or clinical pathways based on the guideline. This advanced functionality can be coupled with the CareSelect benchmarking and reporting tools to ensure success.
The CareSelect platform enables the delivery, management and localization of clinical guidelines that range from simple appropriate use criteria to complex level of care admission level logic. The platform simplifies the configuration of the EMR and leverages all available clinical data to ensure that guidelines are accurately delivered.
For more information: www.nationaldecisionsupport.com

 May 22, 2024
May 22, 2024