June 27, 2013 — MRS (Mammography Reporting System Inc.) has earned the top ranking in the Mammography Information Systems (MIS) vendor category in the newly released KLAS report entitled Women's Imaging 2013: Measuring the Options. The Women’s Imaging 2013 report is an independent overview of industry trends and advancements among key vendors conducted by KLAS, which is an independent research firm that is widely recognized in the healthcare industry.
In the recent KLAS report, MRS is described as, “Leader of the MIS market with solid performance. Reporting is touted as fantastic, including MQSA, critical results, and clinical reports. Flexible, robust functionality for mammography-specific functions and regulatory requirements. Tracking patients for follow-up procedures and yearly screenings is easy with MRS.”
Along with this summary, the report also contains several positive reviews of the product from current users. One user stated, “MRS is a very strong company that gives us excellent service. They are small, so we get a lot of attention.” Another user stated, “We have had some pressure to switch, but there has been much resistance on our end because we love MRS.”
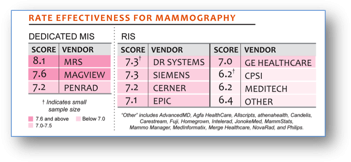
MRS is also highlighted in the key finding section. The report states, “MRS Increases Performance Gap- Overall, women’s imaging providers prefer using a dedicated MIS over a RIS for mammography. MRS is the strongest performer with v.7 adding new functionality.” Overall, women’s imaging providers prefer to use a dedicated MIS to a RIS for mammography.
“We are gratified to see an independent research firm with the stature of KLAS validate what we have been hearing from our customers: that MRS7 is an outstanding product with a great feature set for breast imaging facilities. Our entire team in Washington, Oregon and North Carolina is proud that their hard work has increased the Performance Gap in 2013, as reflected in the KLAS ranking,” said Mark Morris, president/CEO of Mammography Reporting System Inc.
For more information www.mrsys.com, www.KLASresearch.com


 February 18, 2026
February 18, 2026 









