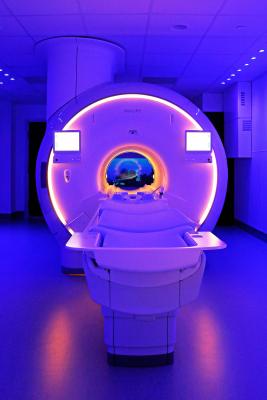
May 17, 2019 ― Miami Cardiac & Vascular Institute announced the implementation of Philips’ Ingenia Ambition 1.5T MR, the world’s first magnetic resonance imaging (MRI) system to enable helium-free operations. The technology offers cutting-edge imaging techniques that deliver high-quality images faster while providing an improved, more comfortable experience for patients, according to Philips.
The Ingenia Ambition incorporates the Philips Compressed Sense acceleration technique, which speeds up 2-D and 3-D scans by up to 50 percent with virtually equivalent image quality, even for challenging patients. The device also features technology that significantly improves patients’ experience during MRI exams, enhancing comfort and compliance. Patients are guided throughout the process by an immersive audio-visual experience, helping them feel at ease and resulting in smoother, faster exams, which contributes to reduced rescans and patient sedation.
"This breakthrough technology means a significant benefit for patients. Those who require an MRI exam, which is often accompanied by feelings of stress and discomfort, can now look forward to a more seamless and pleasant experience,” said Constantino Pena, M.D., medical director of vascular imaging, Miami Cardiac & Vascular Institute. “At the same time, the added speed with uncompromised quality allows us to conduct exams more efficiently, reducing unnecessary wait times that can lead to delayed diagnoses and increased costs.”
The Ingenia Ambition is based on Philip’s BlueSeal fully sealed magnet, which uses a micro-cooling technology that requires a negligible amount of liquid helium (less than 0.5 percent of volume, compared to the Ingenia 1.5T ZBO magnet). This reduces potential helium-related issues of classic magnet designs and eliminates dependency on the scarce commodity. The magnet also employs an adaptive intelligence system aimed at minimizing downtime in the event of operational issues, ultimately allowing practitioners to conduct testing with greater efficiency and confidence.
Miami Cardiac & Vascular Institute offers a range of cardiovascular services and is continuously introducing new and advanced technology to better treat patients and further understand the complexity of disease, injury and congenital or acquired abnormalities.
“The incorporation of Ingenia Ambition represents a significant addition to our robust offering of diagnostic services. With this new capability, we can reinforce the delivery of consistent, high-quality care that leads to better outcomes for patients,” stated Pena.
Read the article "Philips Launches Ingenia Ambition X 1.5T MR"
For more information: www.usa.philips.com/healthcare


 January 28, 2026
January 28, 2026 









