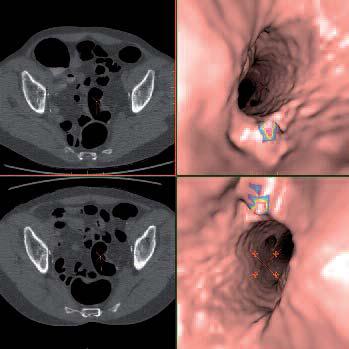
July 15, 2011 — Medicsight PLC, developer of computer-aided detection (CAD) and medical image analysis software, has entered into an agreement with Ziosoft Inc., a pioneer in supercomputing advanced visualization and functional analytics. The agreement includes the integration of Medicsight’s ColonCAD application programming interface (API) software and Ziosoft’s Ziostation technology as well as distribution of both technologies by Ziosoft in the United States. One of the first U.S. sites to use the combined technologies will be the University of Wisconsin School of Medicine and Public Health (UWH) in Madison.
“The partnership between Ziosoft and Medicsight should make a compelling match for CT [computed tomography] colonography interpretation,” said Perry J. Pickhardt, professor of radiology at UHW. “CTC will definitely benefit from the pairing of these companies which are two recognized leaders in the advanced visualization field.”
Medicsight received regulatory clearance for clinical use of its technology in May. Medicsight’s ColonCAD is designed to assist radiologists during their review of CT colonography (CTC – also known as “Virtual Colonoscopy”) images by automatically highlighting potential colorectal polyps (possible precursors to colorectal cancer) on the CT image. The regulatory clearance was supported by a large clinical trial involving 15 radiologist readers who each reviewed 112 patient CTC cases. The clinical trial results demonstrated that, when assisted by ColonCAD, radiologists’ accuracy for detecting polyps of all sizes was significantly improved compared with unassisted reading.
Ziosoft holds numerous clearances for a variety of applications for its supercomputing visualization and functional analytics technology. Medicsight and Ziosoft have been partnering in Europe prior to the U.S. clearance of ColonCAD software.
For more information: www.medicsight.com; www.ziosoftinc.com


 April 18, 2025
April 18, 2025 









