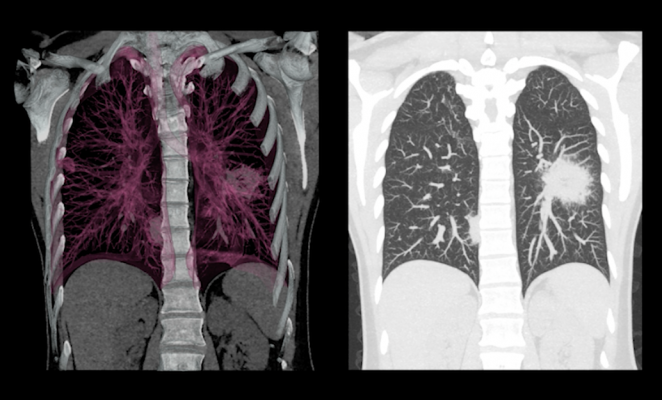
September 13, 2017 — Matrix Analytics announced it will clinically validate its LungDirect deep learning and predictive analytics tools for pulmonary nodules. Matrix Analytics has already developed technology for the management of pulmonary nodules. This technology, leveraged with cloud computing and deep learning, will begin clinical validation later this month. The new tool applies an automated cancer classifier identified in conventional chest computed tomography (CT) images to LungDirect's advanced learning algorithms.
Unlike computer-aided detection (CAD), LungDirect's deep learning tool creates a cancer classifier to guide the algorithm towards a detailed outcome. Deep learning tools have already achieved remarkable diagnostic results, and LungDirect will be the first screening and incidentaloma software to incorporate this technology, according to the company. Matrix Analytics is partnering with top academic centers to combine imaging techniques, machine learning, and deep electronic health record (EHR) integrations to develop big data pipelines that will train LungDirect's platform to achieve superior performance.
For more information: www.matrix-analytics.com


 March 06, 2026
March 06, 2026 









