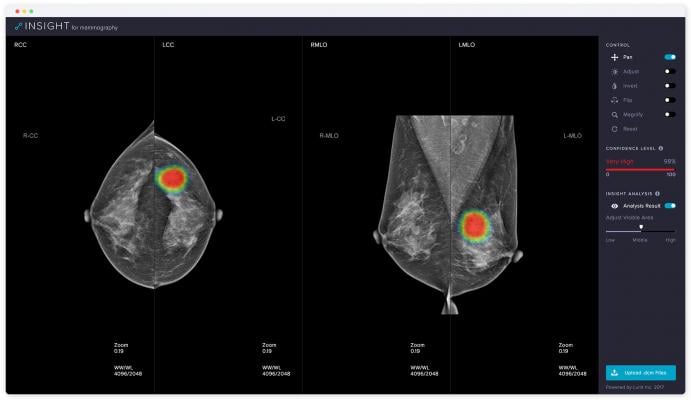
November 15, 2018 — Medical artificial intelligence (AI) software company Lunit will be returning to the 104th Radiological Society of North America (RSNA) annual meeting, Nov.25-30 in Chicago, launching its new solution for mammography—Lunit Insight for Mammography.
Lunit Insight for Mammography is a diagnostic support tool that accurately detects malignant lesions suspicious of breast cancer with 97 percent accuracy. It provides location information of detected lesions in heatmaps and shows abnormality scores that reflect the probability of malignancy. Expected to clear U.S. Food and Drug Administration (FDA) and CE approval for clinical use by late 2019, Lunit Insight for Mammography will open to the public for a free online demo on November 19.
Lunit Insight for Chest Radiography will also be showcased at RSNA. Since being launched during RSNA 2017, it has been tested in more than 1.5 million cases from more than 70 countries. With an accuracy level of 97-99 percent, it detects major chest abnormalities including nodules, consolidation, pneumothorax and more. The computer-aided detection (CAD)-based solution is also being integrated into the worklist for prioritization, in which positive cases may be read first in order to decrease turnaround time and increase overall reading productivity.
Other products currently in research will also be available for demonstrations.
On Wednesday, Nov. 28, Brandon Suh, the new CEO of Lunit, will be on stage at theMachine Learning Theater to give a presentation. Titled, “From AI-powered Diagnostic Support Tools to Imaging Biomarkers: Aiming Beyond Human-Level Accuracy,” Brandon will provide an overview of Lunit’s recent achievements and future developments.
For more information: www.lunit.io


 March 06, 2026
March 06, 2026 









