Lunit's AI-powered chest X-ray analysis solution Lunit INSIGHT CXR
January 16, 2024 — Lunit, a leading provider of AI-powered solutions for cancer diagnostics and therapeutics, announced a three-year supply contract with Samsung Electronics (Samsung Healthcare). The collaboration centers around the integration of Lunit's AI technology into Samsung's premium X-ray devices, elevating the accuracy and speed of chest screening.
Under the terms of the contract, Lunit will supply Samsung Electronics with two AI-powered chest screening solutions: Lunit INSIGHT CXR and Lunit INSIGHT CXR Triage. The initial phase of this collaboration will see the X-ray devices, enhanced with Lunit's AI solutions, targeting the markets in the US, Canada, and Europe. Future phases aim to expand this to the Middle East, South America, and Southeast Asia, thereby broadening the global reach.
In line with the urgency of cases typically encountered in Intensive Care Units (ICUs) and Emergency Rooms (ERs), X-ray devices with the Lunit INSIGHT CXR Triage are expected to be primarily deployed in these high-stakes medical settings.
"The synergy between Lunit and Samsung Electronics will enable faster, more accurate chest screenings, leading to timely interventions and improved patient outcomes. We’re excited about the potential this partnership holds for advancing chest X-ray practices, particularly in ICUs and ERs,” said Brandon Suh, CEO of Lunit.
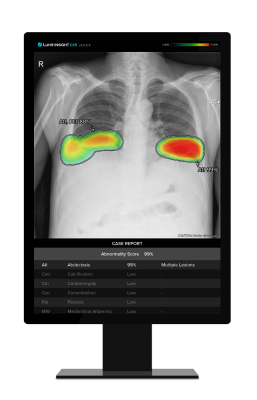
Lunit INSIGHT CXR, an AI-powered chest X-ray analysis solution, stands out for its capability to detect 10 of the most common lung abnormalities, including lung cancer, pneumonia, and pneumothorax. Lunit INSIGHT CXR Triage is an FDA-cleared AI solution that identifies pre-specified critical findings, such as pleural effusion or pneumothorax, on frontal chest X-ray images. The software then flags the images in the Picture Archiving and Communication System (PACS)/workstation, enabling a prioritized review process for timely intervention.
For more information: https://www.lunit.io/en


 February 05, 2026
February 05, 2026 









