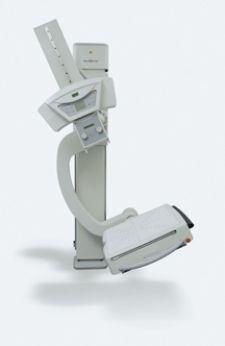
The Kodak DIRECTVIEW DR 3000 recently received 510k clearance from the U.S. Food and Drug Administration (FDA).
The DR 3000 system’s operator console enhances productivity, while the motorized positioning arm enables the efficient capture of a variety of general radiography exams. In addition to capturing upright and table exams, the Bucky’s tilting feature facilitates angled projections. Automatic positioning for upright and table projections, and the constant alignment of the X-ray tube and detector can save time for radiographic technologists and patients.
Preview images are available in less than eight seconds. The detector also features a large 17-by-17-inch image area (43-by-43-cm) that eliminates the need for rotation. The compact system features an optional moveable table and a floor-mounted stand that does not require wall mounts or ceiling suspension provisions. This creates greater placement flexibility and reduces the cost of installation. The system’s large cesium iodide detector matrix (3,121 by 3,121) with 143 micron pixels, combined with Kodak’s proprietary image processing software, produces sharp, detailed images.


 August 14, 2025
August 14, 2025