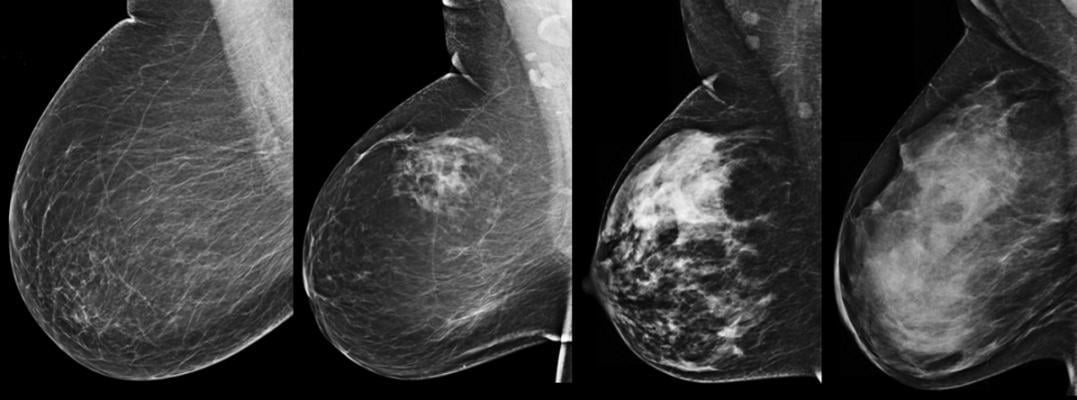
April 14, 2017 — Kentucky and Iowa both this week joined the ranks of states with laws requiring mammography facilities to inform patients about the impact of dense fibroglandular breast tissue and its impact on cancer diagnosis and detection.
Kentucky’s legislation, designated HB78, was signed into law by Gov. Matt Bevin on April 11. Beginning in early July, the law will require mammography providers to notify women whose mammograms reveal dense fibroglandular breast tissue, as determined by the American College of Radiology’s Breast Imaging Reporting and Data System (BI-RADS) classification. The notice must also inform these patients of their slightly increased risk of developing breast cancer, as well as the increased difficulty of catching early cancers due to masking.
"I am honored to support legislation which gives Kentucky women potentially life saving information about their own breast health.,” said. Rep. Jim DuPlessis. “Knowledge is power and knowing ones own risk factors is the ultimate power in keeping yourself healthy. This bill will not only inform patients, it will enhance dialogue with their healthcare providers about their screening options that best fit their needs."
While the language of HB78 does not offer specific suggestions for follow-up screening, the law does encourage providers to recommend digital mammography, including breast tomosynthesis, when writing orders for mammograms.
The Iowa law, designated SF250, was signed by Gov. Terry Branstad on April 13 and takes effect beginning Jan. 1, 2018. Under the terms, all mammography reports will include information about the patient’s individual fibroglandular density as assessed under BI-RADS; patients who are determined to have heterogeneously or extremely dense breasts will be informed of the associated risks.
Kentucky and Iowa became the second and third states to enact breast density inform laws during the first legislative session of 2017, after Colorado did so on April 6.
New Mexico was poised to join the wave with its own HB 243, but the legislation was subjected to a pocket veto by Gov. Susana Martinez, who did not take action on the bill prior to the April 7 deadline. The language of the New Mexico bill was similar to that of Iowa’s law, requiring all patients to be informed of their individual breast density and additional warnings for those with heterogeneously or extremely dense breasts. "As a breast cancer survivor, I feel strongly that all women deserve the best care possible. I will definitely bring the bill before the legislature until it becomes law in New Mexico," said Rep. Liz Thomson.
Nebraska also has a bill in progress, which was placed on final reading in the state Senate on April 10.
For more information: www.areyoudense.org, www.densebreast-info.org


 February 18, 2026
February 18, 2026 









