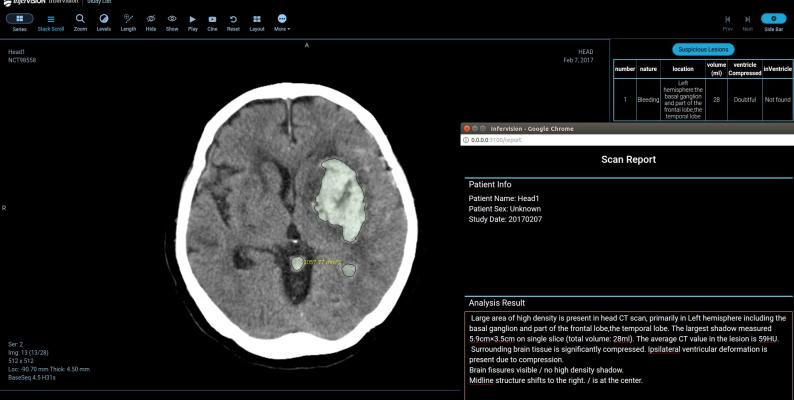
July 8, 2019 — Infervision announced their InferTEST program at the recent Society for Imaging Informatics in Medicine (SIIM) conference, June 26-28 in Denver. InferTEST provides a gratis, no-commitment method for hospitals and radiology groups to see how artificial intelligence (AI) works and what value such tools can add to their practices and patients.
Infervision VP Tony Gevo explained that a sample file is uploaded to the company’s secure FTP headquarters site in Philadelphia. A detailed report of what the InferTEST system found is then sent back to the submitting provider for their review. Gevo added that InferTEST results could also be used in peer review or retrospective screening programs.
According to Infervision, its AI products are currently in use at more than 325 sites across North America, Europe and Asia, assisting in reading more than 33,000 exams daily.
For more information: www.infervision.com


 February 13, 2026
February 13, 2026 









