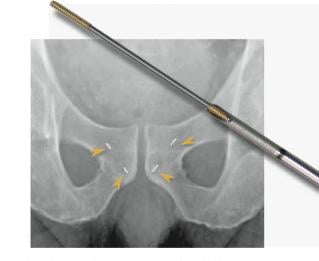
September 24, 2013 — IBA (Ion Beam Applications S.A.) has launched an extension to the Visicoil product line at the annual ASTRO congress of the American Society for Radiation Oncology. This new design incorporates kV X-ray imaging compliant Visicoil Linear Markers pre-loaded in 21 and 22 gauge needles that are almost half the size of needles typically used for placement of gold seeds.
Compared to typical gold seeds, this new design enhances the ability of interventional radiology to safely and efficiently implant Visicoil markers, which subsequently supports radiation oncologists in highly precise patient setup and dose delivery during radiation therapy.
This new solution offers clinical benefits associated with smaller needles, such as increased patient safety and improved patient comfort. IBA’s patented Visicoil linear fiducial markers are an important component in the effort to increase targeting accuracy in radiation therapy applications such as stereotactic body radiation therapy (SBRT), image-guided radiation therapy (IGRT), intensity-modulated radiation therapy (IMRT), CyberKnife and proton therapy.
For more information: www.visicoil.com


 February 27, 2026
February 27, 2026 









