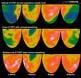
September 21, 2009 – IBA (Ion Beam Applications S.A.), a provider of PET radiopharmaceuticals, and Aposense Ltd., a developer of agents targeting apoptosis (programmed cell death) for molecular imaging and therapy, today announced an exclusive collaboration agreement for commercializing Aposense [18F]–ML-10, Aposense’s novel agent for molecular imaging of apoptosis.
The new agent is a small molecule radiotracer which allows the imaging of apoptosis, a fundamental biological process of controlled cell death, from the early stages of the death process. Given the broad, cross disease role of apoptosis in a wide range of medical disorders, molecular imaging with [18F]-ML-10 is expected to play an important role in early detection of disease, monitoring of disease course, assessment of effect of treatment or development of novel therapies. In particular, [18F]-ML-10 may assist oncologists in evaluating tumor response to treatment much earlier than conventional imaging modalities such as CT or MRI. This may allow clinicians to identify earlier the most effective treatment within their therapeutic arsenal and provide personalized, safer and more cost-effective care. Among other clinical fields of potential applications of [18F]-ML-10 are cardiology and neurology.
[18F]-ML-10 illustrates the capability of nuclear molecular imaging to become a central discipline in healthcare and to deliver the clinical and economic benefits of Personalized Medicine.
The long-term, global agreement includes the collaboration and joint-funding by IBA and Aposense of phase III and subsequent clinical development of [18F]-ML-10. In addition, the companies will jointly market and sell [18F]-ML-10, with IBA primarily focusing on its core PET imaging and nuclear medicine market, and Aposense marketing to the referring clinical specialist market. Aposense will manufacture the proprietary ML-10 precursor and IBA will 18F-label and distribute the final drug product to clinical sites through its global network of PET radio-pharmacies. IBA and Aposense will share in revenue and development costs. Specific financial terms were not disclosed. Multi-center phase II clinical trials of [18F]-ML-10 are underway in a number of leading cancer centers in the United States, and are expected to be completed in 2010, to be followed by phase 3 trials during 2011/2012 for obtaining regulatory approvals.
This agreement expands the relationship established between IBA and Aposense in August 2008, when they entered into an agreement for 18F-labeling and distributing of ML-10 by IBA for Aposense’s multi-center clinical trials.
For more information: www.iba-worldwide.com and www.nst.co.il


 February 13, 2026
February 13, 2026 









