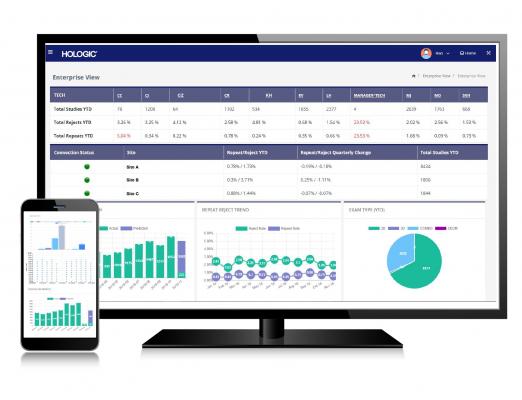
February 12, 2019 — Hologic Inc. announced the U.S. launch of Unifi Analytics, a business intelligence tool that allows healthcare facilities to manage their mammography devices. Users can also monitor technologist performance and prevent unanticipated downtime via predictive tube replacement technology.
Unifi Analytics tracks a facility’s installed base of mammography devices and provides statistical analysis of technology efficiency and technologist accuracy. This allows breast imaging centers to benchmark their performance against similar facilities, identify potential risks and challenges, and maximize device utilization. The web-based platform helps administrators make informed business decisions by providing actionable insights designed to optimize imaging center performance.
Using advanced machine intelligence, Unifi Analytics has the ability to predict tube failures before they occur, allowing facilities to avoid costly downtime. Additionally, predictive procedure volume and quality metrics reports notify facilities if they move off target, giving them the opportunity to make immediate adjustments and potentially stay on top of Mammography Quality Standards Act (MQSA) compliance issues before they arise.
Data collected through Unifi Analytics is distributed through secure, encrypted channels and is de-identified in accordance with Health Insurance Portability and Accountability Act (HIPAA) privacy rules. No personal health information is collected or shared.
Unifi Analytics is only compatible with Hologic mammography systems.
For more information: www.unificonnect.com


 February 18, 2026
February 18, 2026 









