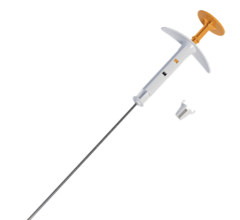
October 21, 2009 - Hologic MammoSite ML (multi-lumen) device offers more women the option of completing their radiation treatment in just five days. Hologic, Inc. offers the MammoSite ML radiation therapy system. With its multi-lumen design, this new device gives radiation oncologists the ability to shape the radiation dose for typical cases and treat patients who are otherwise not appropriate candidates for traditional brachytherapy.
By employing the MammoSite system, the physician can deliver targeted radiation therapy directly to the area where cancer is most likely to recur,(i) allowing a full course of radiation to be delivered in just five days. Additionally, targeted therapy of the breast limits radiation exposure to normal, healthy tissue. This targeting helps minimize side effects such as skin discoloration and scarring, burning, fatigue, and damage to surrounding organs.
The MammoSite systems are comprised of an inflatable balloon catheter in which a radioactive source is introduced for therapy delivery. The inflatable balloon is inserted into the surgical cavity remaining after removal of the tumor. This local placement of the balloon provides for therapeutic delivery of a five-day course of radiation to the tissue most likely to contain residual cancerous cells following surgery, while reducing radiation exposure to adjacent healthy tissue. Using the MammoSite multi-lumen catheter, the radiation oncologist has the ability to shift the radiation dose to the areas that need it most and shift the dose away from areas that do not require it.


 October 01, 2023
October 01, 2023