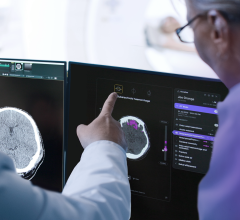
November 17, 2016 — HealthMyne has partnered with Gilda’s Club of Madison (Wis.) in an effort to make a difference in the lives of children and adults living with cancer during the 2016 Radiological Society of North America (RSNA) annual meeting.
For each RSNA attendee that takes part in a demo of HealthMyne’s Quantitative Imaging Decision Support (QIDS) software platform, the company will make a $25 donation to Gilda’s. Gilda’s offers support for those with cancer, but it also provides support for the entire family throughout their entire journey. This includes support groups, lectures, workshops and a variety of social events. Those attendees that take part will have an opportunity to sign their name to the “Donation Board” and get an “I Donated” sticker to proudly display their kind deed.
The QIDS platform combines imaging data with electronic medical record, radiation therapy and other clinical systems information to provide clinical decision support in the primary read. Currently focused on radiology and oncology, with future expansion into other specialties, QIDS (pronounced ‘quids’) is designed to positively impact clinical productivity and prepares radiologists for the transition to value-based payment.
HealthMyne asserts that in order for radiologists to extend their value as a member of the care team, highly curated evidence-based narratives, imaging analytics and patient cohort analysis must be generated to provide clinical decision support in the primary read, thereby enabling improved cancer diagnosis, staging and treatment.
For more information: www.healthmyne.com

 May 22, 2024
May 22, 2024