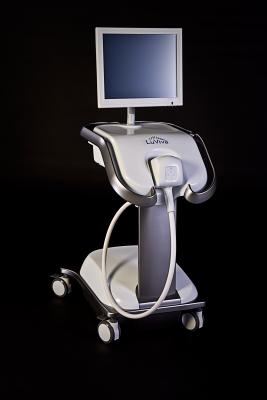
August 18, 2014 — Guided Therapeutics Inc. announced that U.S. Patent and Trademark Office granted a new patent with 22 claims that support the technology behind the LuViva Advanced Cervical Scan.
Patent number 8,781,560 B2, entitled “Method and Apparatus for Rapid Detection and Diagnosis of Tissue Abnormalities,” covers the use of two types of spectroscopy in conjunction with images of tissue to detect abnormalities in tissue. Most importantly, the inventions described in the patent function as an additional bridge from theoretical models of tissue spectroscopy to a fully functional, commercially viable cancer detection system that can be utilized as a platform for many types of epithelial cancers.
“This is an important patent that extends additional protections to key aspects of the underlying technology that make LuViva such a powerful tool in the early detection of disease that leads to cervical cancer,” said Gene Cartwright, CEO of Guided Therapeutics. “Our intellectual property creates a foundation on which we build shareholder value and we will continue to aggressively develop additional patents in the U.S. and worldwide.”
Guided Therapeutics now holds 21 patents with four additional patents pending for its light-based biophotonic disease detection technology.
For more information: www.guidedinc.com


 February 18, 2026
February 18, 2026 









