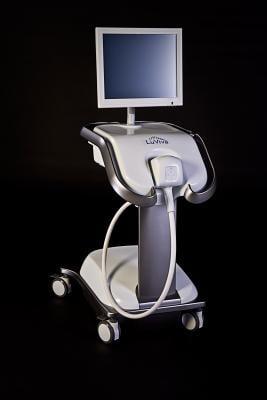
July 30, 2014 — Guided Therapeutics Inc. has filed an amendment to its premarket approval (PMA) application with the U.S. Food and Drug Administration (FDA) for the LuViva Advanced Cervical Scan.
The filing follows the face-to-face meeting the company had with the FDA in May 2014 and addresses questions raised in a Sep. 6, 2013 not-approvable letter that the company received from the agency.
“We believe that our PMA amendment addresses the remaining questions the agency had about our application,” said Gene Cartwright, CEO of Guided Therapeutics. “While we await a decision on the U.S. market, we continue to see momentum building in the larger international market where we are working to increase pilot studies with LuViva, drive sales and build market share.”
The FDA has 180 days to respond to the amendment.
The company has regulatory approval to sell LuViva in Europe with the Edition 3 CE mark, and has marketing approvals from COFEPRIS in Mexico, Health Canada and Singapore Health Sciences Authority, among others. Additionally, expansion efforts are ongoing in the Middle East, Asia and Latin America.
LuViva scans the cervix with light and uses spectroscopy to measure how light interacts with the cervical tissue. Spectroscopy identifies chemical and structural indicators of precancer that may be below the surface of the cervix or misdiagnosed as benign. This technique is called biophotonics. Unlike Pap, HPV tests or biopsies, LuViva does not require laboratory analysis or a tissue sample, and is designed to provide results immediately, which may result in eliminating costly, painful and unnecessary additional testing. LuViva is intended for use with women who have undergone initial screening and are called back for follow up with a colposcopy examination, which in many cases involves taking a biopsy of the cervix. The device is used in conjunction with the LuViva Cervical Guide single-use patient interface and calibration disposable.
For more information: www.guidedinc.com


 February 18, 2026
February 18, 2026 









