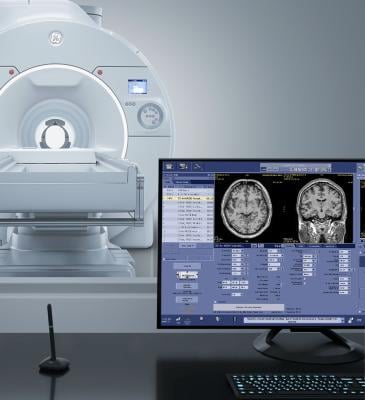
GE HealthCare has announced the unveiling of its SIGNA MAGNUS, an FDA 510(k) pending head-only magnetic resonance (MR) scanner designed to explore advancements in neuroscience. The company launch coincides with the International Society for Magnetic Resonance in Medicine (ISMRM) Annual Meeting, taking place from May 4-9, in Singapore. Image courtesy: Getty Images
May 6, 2024 — GE HealthCare has announced the unveiling of its SIGNA MAGNUS[i], an FDA 510(k) pending head-only magnetic resonance (MR) scanner designed to explore advancements in neuroscience, which have been restricted by the performance limitations of conventional whole-body MR systems. The FDA 510(k) pending scanner is part of GE Healthcare’s presence at the International Society for Magnetic Resonance in Medicine (ISMRM) 2024 ISMRM Annual Meeting, taking place from May 4-9, 2024, in Singapore.
Neuroscience, particularly in the study of psychiatric diseases and neurological disorders such as Alzheimer’s, has been constrained by technological and biological limitations, leaving many aspects of the brain structure and functionality largely unexplored, noted GE Healthcare in a written statement about the unveiling. It added that 43% of the world’s population suffer from neurological disorders[ii], yet only a fraction can be diagnosed by MRI, making the need to expand the neurological clinical applications of MRI critical.
The company also reported that in March 2024, the investigational MAGNUS system was successfully installed at Brigham and Women’s Hospital, a leading research institution, part of Mass General Brigham. The Brigham team will play a crucial collaborative role in performing research on high-performance neuro MR with GE HealthCare, according to the unveiling update. “With this system, we will be able to measure things that weren’t possible with conventional MRI. We can ask questions we couldn’t ask before,” said Carl-Fredrik Westin, PhD, the project’s principal investigator, and founding director of the Laboratory of Mathematics in Imaging and director of the Neuroimaging Analysis Center in the Department of Radiology.
Enhancing Neurological and Oncological Diagnosis and Research
SIGNA MAGNUS represents GE HealthCare’s vision of its most advanced 3.0T MR imaging device, specifically designed for the highest standards of neurological and oncological research for head-only imaging. The product, reports the company, was designed to help to empower neuroscientists, neurologists, neuroradiologists and oncologists to transcend barriers, with the goal of enhancing the diagnosis, understanding, and treatment of complex diseases.
"With SIGNA MAGNUS, we are not just exploring the possibility of providing the tool; we are setting new benchmarks in medical research and future clinical patient care.” said Kelly Londy, CEO, MR GE HealthCare. Londy added, “This innovation underscores our commitment to R&D and our collaborations with academia, pushing the boundaries of what's possible in MR imaging. The potential impact of SIGNA MAGNUS on patient outcomes and our understanding of the human brain is profound."
This advanced system offers superior gradient performance with its HyperG gradient technology, featuring 300 mT/m and 750 T/m/s, enabling the detection of fine details that were previously unattainable. The company statement further offered that researchers can fully utilize SIGNA MAGNUS capabilities to push the boundaries of advanced anatomical, diffusion and functional techniques, amplified by the latest deep-learning algorithms that GE HealthCare has to offer. As such, it added, SIGNA MAGNUS is designed to be a gateway to new research opportunities, helping to uncover new parameters and biomarkers with its vast potential. Its innovative asymmetric gradient design allows for remarkable diffusion performance, achieving extremely high B-value diffusion with short echo times (TEs), which further refines the understanding of neural architecture. Additionally, many GE HealthCare 3.0T systems are upgradable to SIGNA MAGNUS, which will help potential customers to save on capital costs.
More information: www.gehealthcare.com
References:
[i] 510(k) Pending at FDA. Not Available for Sale in the United States. Not yet CE marked. Cannot be placed on the market or put into service until it has been made to comply with CE marking. Not cleared or approved by any global regulator for commercial availability.
[ii] Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. LancetNeurol2024; 23: 344–81
Huang Y, Li YA, Pan HY, Han LY. Global, regional, and national burden of neurological disorders in 204 countries and territories worldwide. J Glob Health 2023;13:04160.


 March 03, 2026
March 03, 2026 









