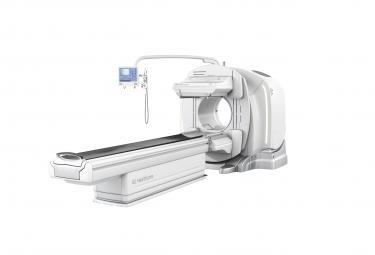
May 5, 2016 — Doctors and researchers at Barnes-Jewish Hospital and Washington University in St. Louis may be able to better diagnose and monitor diseases at a functional level with GE Healthcare’s next-generation SPECT/CT system, Discovery NM/CT 670 CZT. GE claims it is the world’s first general purpose, ultra-high resolution single photon emission computed tomography (SPECT)/CT imaging system, with a new digital detector powered by cadmium zinc telluride (CZT) technology.
SPECT/CT exams are performed to assess the functionality of organs and play a key role in the diagnosis and monitoring of a multitude of diseases. GE Healthcare’s new system is equipped with CZT technology that enables direct conversion of photons into a digital signal, therefore making the technology more efficient. Until now, CZT technology has been limited to organ-dedicated devices, whereas Discovery NM/CT 670 CZT is the first to allow doctors to perform exams on every organ, including whole-body exams.
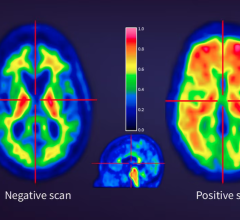
This technology is intended to help improve clinical efficacy and patient experience. Improved efficacy will allow doctors to detect smaller lesions and quantify them more accurately due to the increased spatial and contrast resolution. This may have a significant role in assessing and monitoring responses to therapies. Having the ability to complete multiple scans in a single visit and reduce the dose injected or the scan time by 50 percent will improve patient experience. Optimizing the duration of the exams or the injected dose represents not only an improvement for the patient experience, but also helps increase the operational and financial efficiency of hospitals.
“We were able to do an in-field upgrade to the Discovery NM/CT 670 CZT, which was a faster and more efficient way to enable us to leverage the new CZT detector. We believe this new system will allow us to see improvements in lesion detectability and will increase the utility of SPECT/CT; we will also look to see how we can reduce scan time, lower dose or both, while maintaining image quality,” said Barry A. Siegel, M.D., professor of radiology and medicine, senior vice-chair and division director of nuclear medicine at Mallinckrodt Institute of Radiology at Washington University in St. Louis. “We look forward to doing research on SPECT/CT quantification, as accurate and reproducible quantification will be increasingly important in nuclear medicine.”
Researchers have identified clinical scenarios where the combination of multiple SPECT tracers could aid physicians in diagnosing and giving much better and more specific reports in difficult patient conditions. However, performing such multi-isotope exams is quite challenging on conventional cameras. Multiple isotope exams could offer a greater insight into the diagnosis and monitoring responses to treatments. With the Discovery NM/CT 670 CZT, clinicians will be able to simultaneously visualize and analyze multiple physiological processes in a patient, gaining insights into multiple dimensions of the patient’s anatomy and physiology at the same time using the hybrid SPECT/CT.
For more information: www.gehealthcare.com


 February 03, 2026
February 03, 2026