November 21, 2013 — Based on its recent analysis of the wireless capsule
endoscopes market, Frost & Sullivan presented Check-Cap Ltd. with the 2013 European Frost & Sullivan Award for New Product Innovation Leadership.
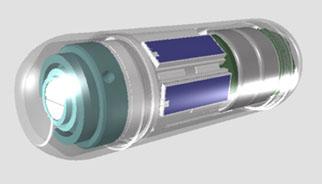
Check-Cap's capsule device, also named Check-Cap, is based on an
imaging approach that enables the 3-D virtual reconstruction of the colon, eliminating the need for bowel preparation. The imaging capsule, which resembles a vitamin capsule in its size and shape, is easy to swallow and is designed to wirelessly transmit data to a small receiver that is no bigger than a disposable patch on the patient back.
Wireless capsules need to satisfy requirements of both patients and physicians. From the perspective of the patient, the capsule has to do away with, or at least significantly ease, the most bothersome aspect of the current screening procedure: bowel preparation. They also have to be small enough for easy oral administration and unhindered mobility through the gastrointestinal track. From the physician's perspective, it should provide 360-degree visualization and cover the colon comprehensively in both quality and quantity. Check-Cap meets all aforementioned requirements.
"While competing products strive for a 360-degree view of the colon by adopting multiple cameras and either fall short or risk image overlaps and redundancy, Check-Cap has adopted an X-ray platform that not only enables a 360-degree view, but also obtains information that is 'hidden' or inaccessible to other products that employ rays of visible light," said Bhargav Rajan, research analyst, Frost & Sullivan.
Although wireless capsule endoscopes are more comfortable for the patient than traditional endoscopes, they suffer from incomplete imaging of the colon due to lack of maneuverability. To combine the best of both wireless and traditional endoscopes, Check-Cap uses X-ray radar technology, which emits X-rays through the device's surface in all directions. The X-ray data that is backscattered and captured by the capsule detectors is then converted, post-procedure, to 3-D images of the colon's internal surfaces. This feature allows physicians to perform the procedure without an uncomfortable and prolonged colon cleansing procedure and detect polyps that are not visible to other capsule endoscopes.
Check-Cap is currently undergoing clinical trials in Europe and is planning to submit the request to initiate an efficacy study in North America late next year. Like other routine radiographic diagnosis of the gastrointestinal (GI) tract, Check-Cap requires a barium-swallow pre-procedure to help differentiate the colon structure from the content.
The X-ray radar technology also maximizes the critical diagnostic time available to the physician, while the 3-D reconstruction of the colon facilitates accurate diagnosis. Check-Cap not only eliminates the redundancy caused by multiple cameras, but also ensures that there is little or no chance of images being left out or not captured through insufficient coverage.
"Check-Cap estimates a review time of seven to 10 minutes to be sufficient for physicians to identify clinically significant polyps and prescribe a follow-up accordingly," said Rajan. "X-ray radar technology not only differentiates the Check-Cap product from its competitors, but is also designed to match classical colonoscopy in its clinical accuracy."
Each year, Frost & Sullivan presents this award to the company that has developed an innovative element in a product by leveraging leading-edge technologies. The award recognizes the value added features/benefits of the product and the increased return on investment (ROI) it offers customers, which in turn increases customer acquisition and overall market penetration potential.