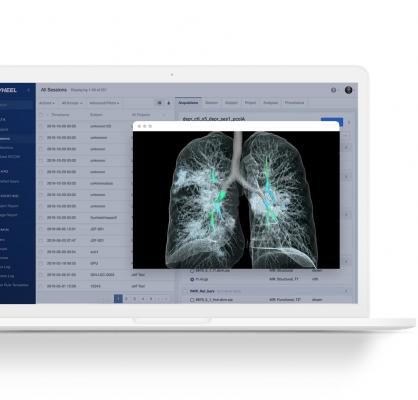
May 7, 2021 — Flywheel Exchange, Inc., a leading bioinformatics platform for cloud-scale data management and collaboration, and Imbio, a leading provider of artificial intelligence (AI) solutions for medical imaging analysis, have partnered to accelerate lung disease discovery, diagnosis, and treatment in the Life Sciences. The Imbio FDA cleared and research-dedicated imaging biomarkers will now be offered on Flywheel's platform.
Researchers today leverage Flywheel for imaging research, AI development, and multi-site collaboration in the academic, clinical and life science markets. Flywheel's comprehensive, cloud-scale platform streamlines data management, standardizes image processing, and enables machine learning for research. With Flywheel, life sciences organizations can improve efficiency in their R&D processes, accelerate innovation and shorten clinical trial timelines.
Through the use of AI, machine learning and deep learning, Imbio algorithms transform the way patients are discovered, diagnosed, treated, and managed, enabling more personalized care. Imbio delivers a suite of fully-automated lung and cardiothoracic algorithms that rebuild grayscale computed tomography (CT) images into rich visual maps with quantitative reports of patient conditions. Pharmaceutical companies on the Flywheel platform can instantly gain access to regulatory cleared analyses and use Imbio's expertise in imaging biomarkers to enhance drug development.
"Flywheel enables us to deliver our products to Life Sciences researchers seamlessly. Researchers can leverage our biomarkers for large-scale initiatives ranging from exploratory research to supporting clinical trials. Flywheel can also support Imbio's collaborations with pharmaceutical companies in the development of customized companion diagnostics," stated Dave Hannes, CEO of Imbio.
With the combined Imbio and Flywheel solution, large volumes of curated data can be processed on the cloud by FDA cleared and FDA pending imaging biomarkers for better understanding of disease diagnosis and progression. These algorithms applied to clinical trials can improve cohort enrichment and patient baselining, and provide quantitative endpoints to assess therapy response. Jim Olson, CEO of Flywheel, stated, "We are excited to partner with companies that share our vision. Imbio's impressive list of imaging biomarkers and Flywheel's automated data organization and cloud-scale processing capabilities are a natural fit in supporting lung imaging research."
For more information: https://flywheel.io/


 February 16, 2026
February 16, 2026 









