Provision Center for Proton Therapy is helping pioneer a new product that will help further protect prostate cancer patients from the effects of radiation therapy. Four patients were injected with SpaceOAR hydrogel, the first product in the United States cleared just last week as a spacer to protect the rectum in men undergoing radiation therapy for prostate cancer. The SpaceOAR System is intended to temporarily position the anterior rectal wall away from the prostate during radiotherapy for prostate cancer, creating space to protect the rectum from radiation exposure. Provision is the first proton therapy center and the second radiation treatment center nationwide to adopt the product.
“We’re pleased to be the first proton therapy center to introduce this additional protection for our cancer patients,” said Marcio Fagundes M.D., radiation oncologist and medical director for Provision Center for Proton Therapy. “By its nature, proton therapy’s targeted radiation dosage protects surrounding tissues from damage. The SpaceOAR product provides us with even more ability to keep our patients comfortable and further prevent long-term side effects as a result of their treatment.”
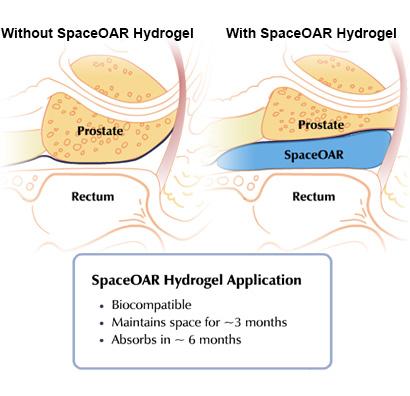
Because of the close proximity of the prostate to the rectum, radiation therapy can cause damage, resulting in long-term side effects. The SpaceOAR system, developed by Augmenix, creates space to protect the rectum from radiotherapy. Placed through a small needle, the hydrogel is administered as a liquid, but quickly solidifies into a soft gel that expands the space between the prostate and rectum. The hydrogel spacer maintains this space until radiotherapy is complete. The spacer then liquefies and is absorbed and cleared from the body in the patient’s urine.
Gary Pitts, a retired urologist from Boone, N.C., was one of the four patients to receive SpaceOAR hydrogel on Tuesday. He came to Provision after one of his patients was successfully treated for prostate cancer with proton therapy several years ago.
“Dr. Fagundes had mentioned the new SpaceOAR hydrogel to me, but we didn’t think it would be approved by the FDA in time,” Pitts said. “He immediately called when he found out it was cleared and I am thrilled to be the first proton therapy patient to receive the extra protection provided by the hydrogel.”
“For years, hydrogel products have been used safely to protect the most sensitive parts of the body as sealants and adhesion barriers, and now prostate cancer patients will also be able to benefit,” said John Pedersen, Augmenix chief executive officer. “FDA clearance of the SpaceOAR System represents a significant development in advancing the safety, precision and flexibility with which prostate cancer radiotherapy can be delivered.”
According to the American Cancer Society and the National Cancer Institute, prostate cancer is second only to skin cancer as the most frequently diagnosed cancer in men with an estimated 220,800 new cases and 27,540 deaths in the U.S. in 2015 alone. Worldwide, prostate cancer is expected to grow to 1.7 million new cases and 499,000 deaths by 2030.
FDA clearance was granted following completion of the SpaceOAR System prospective, multicenter, randomized clinical trial. SpaceOAR patients experienced a significant reduction in rectal radiation dose and severity of late rectal toxicity when compared to control patients who did not receive SpaceOAR hydrogel. The full pivotal clinical trial results have been submitted for publication in a peer-reviewed journal and are expected to be published later this year.
For more information: www.augmenix.com


 January 30, 2026
January 30, 2026 









