Image courtesy of Volpara Solutions
November 12, 2015 — Volpara Solutions announced that it has received a new 510(k) clearance from the U.S. Food & Drug Administration (FDA) for its VolparaDensity breast imaging software.
Originally cleared for use by the FDA in 2010, the new clearance covers VolparaDensity version 3.1, which has been specifically designed to correlate to the new Fifth Edition of the Breast Imaging-Reporting and Data System (BI-RADS) Atlas recently issued by the American College of Radiology (ACR). The 5th Edition Atlas was updated to require “an overall assessment of the volume of attenuating tissues in the breast, to help indicate the relative possibility that a lesion could be obscured … and that the sensitivity of examination thereby may be compromised by dense breast tissue.” Development of Volpara’s new software included a reader study which was used to optimize VolparaDensity performance to the BI-RADS 5th Edition.
Volpara will showcase VolparaDensity 3.1 software and other new advances to its suite of quantitative breast imaging tools at the 2015 Radiology Society of North America (RSNA) meeting, Nov. 29-Dec. in Chicago.
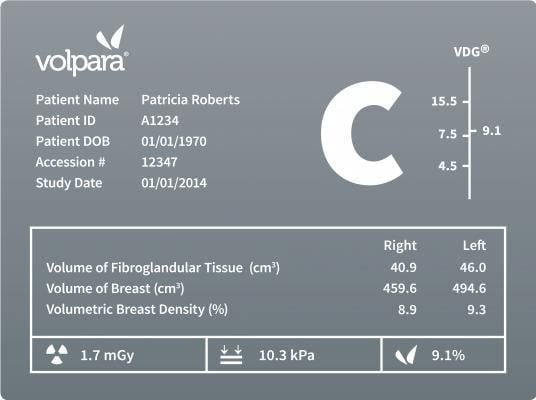
Because of its volumetric approach, VolparaDensity goes beyond measurement of dense tissue area, and actually measures the compressed thickness and volume of dense tissue to truly show when small regions of “focal density” present a masking risk. VolparaDensity results have been clinically validated in independent studies to correlate to mammography sensitivity.
Volpara has also developed a detailed discussion of the changes that have been made to VolparaDensity to reflect the new ACR BI-RADS Atlas 5th Edition, including significant input from the Volpara customer base, study of the literature, reader study results and research using in-house Volpara databases.
Breast density has not only been linked to an increased risk of breast cancer, it also dramatically impacts early detection. Several large studies have confirmed that as density increases the accuracy of mammography decreases. According to a study published in the New England Journal of Medicine, 35 percent of breast cancer goes undetected by mammography in women with dense breasts and density masks appearance of tumors. Since both dense breast tissue and cancer appear white on a mammogram, it is analogous to looking for a snowball in a snow storm.
Cleared by the FDA, HealthCanada, the TGA and CE-marked, VolparaDensity helps radiologists objectively assess density from digital mammography and tomosynthesis images to determine which women might benefit from additional imaging. Highly correlated to breast magnetic resonance (MR) assessments, VolparaDensity automatically generates an objective measurement of volumetric breast density correlated to the American College of Radiology (ACR) BI-RADS breast density categories.
For more information: www.volparasolutions.com


 February 06, 2026
February 06, 2026 









