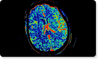
December 15, 2008 - Visage Imaging received FDA 510(k) clearance to market its latest thin-client product release, Visage CS 3.1, providing an upgrade to its prior offerings in cardiac and other tools, and includes new optional neuroradiology and oncology applications.
The Neuro option facilitates brain perfusion analysis in CT and MRI imaging, while the Oncology option provides tools for analyzing, documenting and comparing lesions for multiple modalities, including standardized update value (SUV)-based analysis for PET/CT.
In addition to the new application options, Visage CS 3.1 features numerous new and streamlined measurement and post processing tools such as advanced 3D segmentation, ROI-based analysis and time-value curves, and improved editing of cardiac LV models.
For more information: www.visageimaging.com


 April 18, 2025
April 18, 2025 









