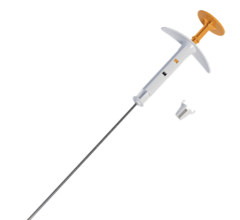
March 5, 2009 – Xoft Inc. received clearance from the FDA for a skin and surface treatment applicator for use with the Axxent Electronic Brachytherapy (eBx) System to deliver surface brachytherapy, including intraoperative radiation therapy (IORT).
This latest FDA Clearance allows the applicator to be used on any external or internal surface of the body where radiation therapy is indicated. Previously cleared for accelerated treatment of early stage breast cancer and endometrial and rectal cancers, the Axxent System is also cleared for use in the treatment of surface cancers or conditions where radiation therapy is indicated. The Electronic Brachytherapy treatment platform is designed to deliver localized, nonradioactive, isotope-free radiation treatment in minimally-shielded clinical settings under the supervision of a radiation oncologist.
In addition to skin indications, the FDA clearance also covers surface indications, which means that many intraoperative radiation therapy procedures can be performed with the Xoft source, while surgery is being performed. Intraoperative treatment potentially reduces the time required for radiation therapy by delivering it immediately during surgery before any remaining cancer cells have a chance to grow. IORT, utilizing the Axxent System’s miniaturized X-ray source that can deliver localized and targeted radiation treatment, is designed to minimize radiation exposure to surrounding healthy tissue and enables physicians and treating professionals to remain in the operating room with the patient.
As a platform technology, the Axxent System addresses a variety of oncological and nononcological indications. The Axxent System is designed to deliver nonradioactive therapy directly to cancer sites with minimal radiation exposure to surrounding healthy tissue. Designed to deliver electronic, X-ray-based radiation treatment, the proprietary Axxent treatment platform can be used in many clinical settings. Treatment can be performed without the need for a shielded room, allowing the radiation oncologist and other medical personnel to be present during treatment delivery.
For more information: www.xoftinc.com


 October 01, 2023
October 01, 2023