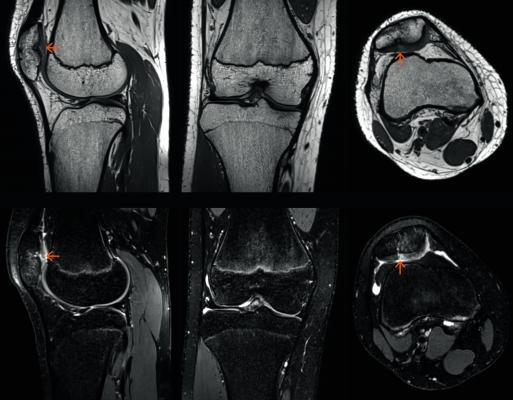
February 19, 2018 — The U.S. Food and Drug Administration (FDA) has cleared GOKnee3D, a magnetic resonance imaging (MRI) application from Siemens Healthineers that significantly reduces the time required to perform comprehensive diagnostic exams of the knee.
GOKnee3D enables a push-button diagnostic 3-D knee exam in just 10 minutes – a substantial reduction from the standard MR knee examination.[1] Acquisition of high-resolution isotropic 3-D images enables flexible evaluation of these images in all planes, including double oblique and curved planar.
The volume acquisition of GOKnee3D enables higher scan speeds and optimal image reconstruction with better signal quality than in previous technologies. Supported by dedicated, high-channel Tim 4G knee coils as well as automated field of view adaptation based on machine learning and artificial intelligence, the MRI scanner acquires volume data of the knee joint with the push of a button.
GOKnee3D is available for the Magnetom Aera 1.5T and the Magnetom Skyra 3T MRI scanners, with eventual rollout planned for additional scanners in the company’s MRI portfolio.
For more information: www.usa.healthcare.siemens.com
Related GOKnee3D Content
Siemens Healthineers Introduces GOKnee3D MR Application
VIDEO: Editor's Choice of the Most Innovative New Imaging Technology at RSNA 2017
Notes
1. Examination time for 3T systems is 10 minutes; for a 1.5T system, up to 11 minutes. All given examination times are examination only; adjustments have been excluded. Applies to measurements only with 15channel knee coil.


 March 03, 2026
March 03, 2026