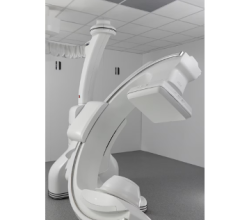
January 14, 2021 — Siemens Healthineers has announced the Food and Drug Administration (FDA) clearance of the Cios Flow, a mobile C-arm designed for use by multiple disciplines in the operating room (OR) – including orthopedics, trauma surgery, spinal surgery, vascular surgery, and pain therapy – to increase the ease and efficiency of everyday imaging workflows for surgical interventions. This multidisciplinary functionality helps to ensure high-capacity system utilization, making the Cios Flow cost-effective. Additionally, its features enable easier, more efficient operation to improve patient care in the OR.
The Cios Flow’s low weight, maneuverability, and intuitive touch-gesture interface simplify operation. When the user taps a challenging anatomical area on the preview image of the Touch User Interface, the SpotAdapt function automatically optimizes relevant imaging and post-processing parameters such as brightness and contrast.
Additionally, the Cios Flow offers Windows 10 security functions to help minimize cyberattacks targeting the system or patient data, as well as Access & User Management features to prevent unauthorized access and restrict sensitive data. Applications can be whitelisted to ensure that the user accesses only safe software from trusted sources. Patient data can be encrypted using BitLocker, and system changes can be tracked.
“With the Cios Flow, Siemens Healthineers proudly offers customers a mobile C-arm system with broad multidisciplinary functionality as well as a robust level of cybersecurity to help enable secure, efficient operations in the OR,” said Lara Barghout, Senior Vice President of Advanced Therapies at Siemens Healthineers North America.
For more information: www.siemens-healthineers.com


 March 06, 2026
March 06, 2026