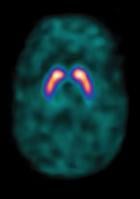
A normal brain scan with the SPECT imaging agent DaTscan showing normal dopamine transporter (DaT) activity.
January 19, 2011 – The U.S. Food and Drug Administration (FDA) cleared a radiopharmaceutical agent intended for use with single photon emission computed tomography (SPECT) imaging, for the detection of dopamine transporters (DaT) in the brains of adult patients with suspected Parkinsonian syndromes (PS).(1)
GE Healthcare’s DaTscan (ioflupane I 123 Injection) is the first FDA-approved diagnostic imaging agent to help physicians evaluate neurodegenerative movement disorders, such as idiopathic (of unknown cause) Parkinson's disease (PD). DaTscan may be used as an adjunct to other diagnostic evaluations to help differentiate essential tremor from tremor due to PS. DaTscan cannot differentiate between the different types of PS.
“Ioflupane may be an important new imaging agent for physicians in differentiating diseases such as Parkinson’s disease from essential tremor,” said Mark Stacy, M.D., neurologist and director of the Duke Movement Disorders Clinic at Duke University Medical Center in Durham, N.C. “Understanding exactly what you are dealing with is important in selecting the appropriate treatments for patients with movement disorders.”
The FDA's action, following a priority review, marks the approval of the first radiopharmaceutical agent to detect DaT distribution within the brain (dopamine is a brain chemical that is classified as a neurotransmitter and is found in regions of the brain that regulate activities such as movement and emotion(2)). The FDA granted DaTscan priority review due to an unmet clinical need for an imaging agent to assist physicians in managing patients according to their dopaminergic status.
“Currently, movement disorders are diagnosed through clinical examinations, blood tests and neuropsychological evaluations, which are not conclusive and may lead to misdiagnosis,” Stacy said. “A new diagnostic adjunct to existing clinical assessments represents a meaningful contribution to the movement disorders field.”
The FDA's approval of DaTscan was based on two phase 3 clinical trials confirming the efficacy of DaTscan for the visualization of DaT distribution within the striata, an interior part of the brain. These studies, evaluating 284 adult patients with tremor, demonstrated the consistent performance of DaTscan in the visual detection of DaT distribution in the brain when compared with a reference clinical diagnosis.(1)
For more information: www.DaTscan.com
References:
(1) DaTscan (Ioflupane I 123 Injection) prescribing information. GE Healthcare, 2010.
(2) Dopamine. Gale Encyclopedia of Medicine. Farmington Hills, MI: The Gale Group Inc., 2008. http://medical-dictionary.thefreedictionary.com/dopamine. Accessed Oct. 19, 2010.


 January 27, 2026
January 27, 2026