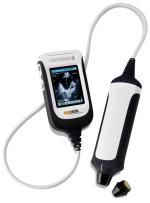
May 21, 2009 – The FDA gave 510(k) clearance to Signostics to market Signos, the company’s portable ultrasound device that weighs just one pound.
The system is one of the smallest and lightest ultrasound devices, the company says. Its high-resolution images are specifically tailored for emergency medicine, primary care, remote healthcare, critical care and sports medicine applications.
For years physicians have been searching for an alternative to the stethoscope for the physical exam. Existing ultrasound systems, even so-called portable systems, consumed too much power, were too expensive and were too large to be carried by physicians, the company says.
By combining technology from modern avionics navigation and modern medical imaging technology, Signostics has developed a high-quality, palm-sized, ultrasound system. It is so simple to use that most clinicians can learn how to capture images in less than an hour, Signostics says.
The system's transducer integrates micro technology and position sensing technology to produce reportedly high-resolution images equal to conventional ultrasound systems without the need for an array or motor.
For more information: www.signosticsmedical.com


 February 05, 2026
February 05, 2026 









