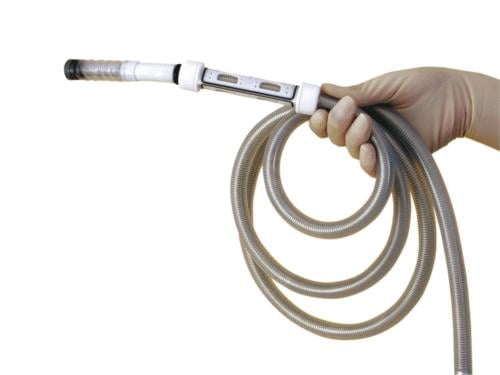
February 24, 2012 — Invendo Medical announced today that the company received 510(k) clearance by the U.S. Food and Drug Administration (FDA) for the company's new C20 colonoscopy system including the SC20 single-use colonoscope.
The Invendoscope SC20 has several features new to the field of colonoscopy:
- It is a single-use colonoscope with a working channel;
- It is not pushed or pulled, but uses a computer-assisted gentle drive technology;
- All endoscopic functions are performed using a handheld device; and
- It reduces forces on the colon wall.
A clinical trial with the company's colonoscope delivered convincing results with a more than 98 percent cecal intubation rate and lesions detected in 41 percent of screening subjects. No device-related adverse events were observed during the study.
The C20 colonoscopy system has already received the CE mark in Europe.
"We are very proud to have achieved the most significant milestone in our company's history so far," said Berthold Hackl, CEO of Invendo Medical. "We are extremely excited to be bringing this new colonoscopy system to the U.S. market. We believe that the invendo system reduces the burden associated with current conventional systems and that it may help to improve colonoscopy compliance rates, one of the key factors affecting the future incidence of colorectal cancer."
For more information: www.invendo-medical.com


 February 06, 2024
February 06, 2024 









