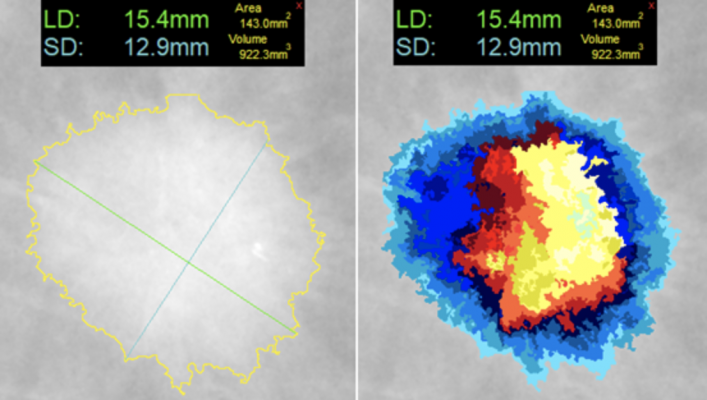
At left, one-click segmentation and measurement rendered by DL Precise. At right, DL Precise uses vivid color to illustrate segmentation.
July 9, 2021 — The FDA has granted 510(k) clearance for DeepLook, Inc.’s DL Precise, a one-click software tool to automate segmentation and measurement of suspicious objects across all medical imaging modalities, delivering critical improvements in workflow and reporting precision.
Leveraging deterministic algorithms rather than artificial intelligence (AI), DL Precise ensures consistent, reliable results. It instantly renders segmentation by contouring an object’s margins and then measures long and short dimension axes, area and estimated volume. DL Precise’s single-click activation eliminates the complex manual setup that characterizes even semi-automatic segmentation tools today and adds important data to AI region-of-interest findings. Users, however, retain full control and can modify any automated measurement as desired.
DL Precise is the first of a series of medical imaging products under development by DeepLook Inc., a Connecticut-based software firm founded by a team with a 20-year track record of success with similar patented image analysis technologies in other industries. Future products will leverage the radiomic data derived from DL Precise’s patented shape-recognition technology that enable automated measurement.
While imaging technology has advanced profoundly, radiologists still routinely use mouse-driven calipers to estimate the size of suspicious objects – and variability continues to undermine the results. Moreover, manual measurement is tedious and time-consuming, requiring multiple mouse clicks and drags, hundreds of times each day. Consistent, accurate measurement is essential to assess growth or reduction of suspicious objects over time, a recurring need in both clinical and research settings.
DL Precise’s entry into the emerging marketplace for a consistent measurement tool with its deterministic algorithm helps ensure reliable performance for today’s workflow demands. The company’s future decision-support and diagnostic products will leverage this core technology to offer key radiomic metrics and enhance AI/machine learning applications. At the same time, DeepLook’s vivid color enhancement helps radiologists visually assess the software’s quantification.
While other measurement products are trained on particular imaging modalities, organs or types of nodules, DeepLook’s patented technology applies to any medical image – a universal utility for mammography, ultrasound, CT and MRI. DL Precise’s automated data can feed directly into structured reports, allowing for easy comparisons even across acquisition modalities – for instance, comparing mammograms to supplemental ultrasound images.
DL Precise is an affordable plug-in utility that works on any DICOM image and in all 510(k)-cleared viewers. Integration with PACS partners is simple, adapted to each viewer interface. End users can master the mechanics in minutes. DL Precise has successfully completed its first integration with the mammography workstation of Three Palm Software, a respected industry innovator.
For more information: www.deeplookmedical.com/dl-precise


 February 16, 2026
February 16, 2026 









