May 9, 2007 - St. Jude Medical, Inc. announced FDA clearance for the EnSite System Version 7 software and EnSite Fusion Registration Module, new products designed to help physicians create more detailed images of the heart for navigating during electrophysiology (EP) procedures that treat complex arrhythmias, which it will feature at the 2007 Heart Rhythm Society meeting on May 9 through 12.
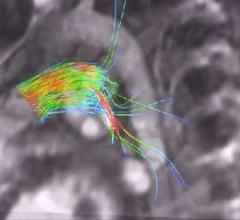
The EnSite System provides highly detailed, three-dimensional (3-D) cardiac models that reportedly help physicians diagnose and treat many abnormal heart rhythms, including atrial fibrillation.
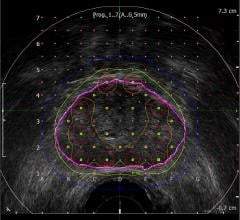
St. Jude Medical's EnSite Fusion software registers, or "fuses," an EnSite-created geometry model with a three-dimensional CT model so that the physician can map directly on the CT image. The result is a 3-D, color, highly-detailed image of a chamber of the heart in which physicians can navigate EP catheters, display electrical information and effectively guide therapy delivery. EnSite Fusion allows physicians to use one system - EnSite - to produce state-of-the art imagery.
EnSite Fusion performs dynamic registration that matches the two models by creating anchor points on the EnSite images which are then linked to points on the 3-D model of the patient’s CT. Fusing the geometric- and CT-derived models gives physicians a more detailed image of the heart to help them deliver ablation therapy.
The new Version 7 software’s field scaling feature is desgined to allow the system to compensate for impedance variations and improve the visualization of the heart’s anatomy and EP catheters. EnSite 7 also includes new measurement tools, which provide additional information about the size and location of key structures in the heart.
The fusion tool also displays electrical information such as voltage, activation timing and lesion data directly on the CT model. In addition, EnSite Fusion provides the flexibility to transition quickly between the fused model and the original EnSite model throughout the procedure, helping clinicians react to changes in patient anatomy or other variations.
“I now can have a better diagnostic tool because two images - a CT image and the cardiac mapping image - are fused into one,” said Richard Schilling, M.D., of St. Bartholomew’s Hospital in London.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now


 July 29, 2025
July 29, 2025