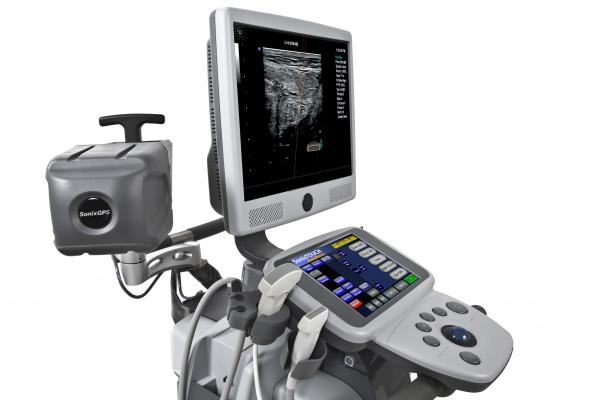
July 11, 2012 — Ultrasonix Medical Corporation has received approval from the U.S. Food and Drug Administration (FDA) for its SonixGPS technology for vascular access procedures.
First approved for use in Canada, the SonixGPS technology has been used in hospitals in British Columbia and Ontario since October 2011. Clinical studies have proven the accuracy and clinical benefits of using the new technology for guidance procedures such as nerve blocks for regional anesthesia and pain management and vascular access procedures. The guidance technology is also useful for difficult biopsies, aspirations and placement of therapeutic agents.
SonixGPS helps clearly predict and see the needle’s trajectory during invasive procedures. Unlike other guidance technologies, SonixGPS enables clinicians to position the transducer in the ideal location to see the target and to select a needle direction and angle that is safest and most comfortable for the patient. It always shows the predicted trajectory and true path of the needle in real time. Needle adjustments are reflected instantly on the display.
SonixGPS has received CE mark for both nerve blocks and vascular access. SonixGPS for nerve block procedures is pending FDA 510(k). The SonixGPS option is available on the SonixTouch, SonixTablet and SonixMDP ultrasound systems.
For more information: www.ultrasonix.com/gps


 February 05, 2026
February 05, 2026