September 26, 2011 — Elekta will highlight several of its radiation therapy products at the American Society for Therapeutic Radiology and Oncology (ASTRO) annual meeting Oct. 2-6 in Miami Beach, Fla.
During the meeting, the company will feature two solutions in particular: Clarity with Autoscan real-time soft tissue tracking and the Identify radiofrequency identification (RFID)-based solution.
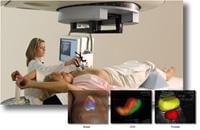
Clarity with Autoscan uses non-ionizing ultrasound technology to continuously track prostate anatomy during simulation and treatment without requiring additional non-therapeutic radiation dose. Unlike other technologies, it does not require invasive implants; images are acquired by placing a probe against the patient's perineum, minimizing any interference or collision risks with the treatment machine. This enables clear visualization of target and critical structure location at any time during treatment delivery and facilitates high precision treatments. As an automated, user-independent imaging technique, the system provides real-time soft tissue tracking without negative impact on treatment times or clinical workflow.
Identify is designed to ensure patient safety in the clinic and increase staff confidence in the reliability of patient identification and accessories. The system employs advanced RFID technology to ensure the right patient is being treated at the right location and with the correct set up and equipment. Identify is integrated with Elekta's Mosaiq Oncology Information System, enabling patient queuing, automatic opening of patient charts and treatment tracking at the EMR, optimizing workflow and safety.
The Elekta exhibit will also feature a live theater at which speakers will give hourly presentations on the company’s solutions, including Identify and Clarity with Autoscan.
For more information: www.elekta.com


 February 03, 2026
February 03, 2026