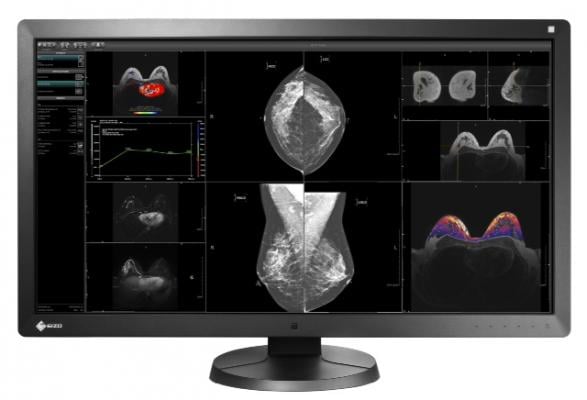
RadiForce RX850 image courtesy of Eizo
October 1, 2015 — Eizo will exhibit two mammography-specific display monitors and the RadiNET Pro network quality control server management software at the 2015 Radiological Society of North America (RSNA) annual meeting.
The RadiForce RX850 high brightness color, multimodality monitor with U.S. Food and Drug Administration (FDA) 510(k) clearance for mammography is specifically designed for all modality workflow needs including digital mammography. A recent study conducted by the University of Arizona shows RadiForce RX850 reduced overall time spent viewing images by 7 seconds and reading time per image by 10 percent, while demonstrating consistent diagnostic accuracy compared to a 5-megapixel monitor.
The RadiForce GX540 features high resolution of 5 megapixels (2048 x 2560) and an LCD LED backlight with fast response time designed specifically for displaying breast screening images. To detect the smallest structures, the monitor offers the highest-in-class contrast ratio of 1200:1. The deeper black levels distinguish similar shades of gray for sharper monochrome image reproduction. Eizo's RadiCS UX1 quality control tool performs precise calibration conforming to DICOM Part 14 and enables quality control complying with American College of Radiology (ACR) Practice Guidelines for digital mammography monitors.
The RadiNET Pro software gives picture archiving and communication system (PACS)/information technology (IT) departments the flexibility to choose the deployment option that matches the needs of their organization. RadiNET Pro features secure, scalable options for both cloud and on premise, with fully centralized remote monitor image quality assurance and compliance with AAPM/ACR as well as any custom quality policy. The PC-independent self-QC (quality control) launches the scheduled task on time regardless of PC status, if it is turned off or not.
For more information: www.eizo.com


 February 18, 2026
February 18, 2026 









