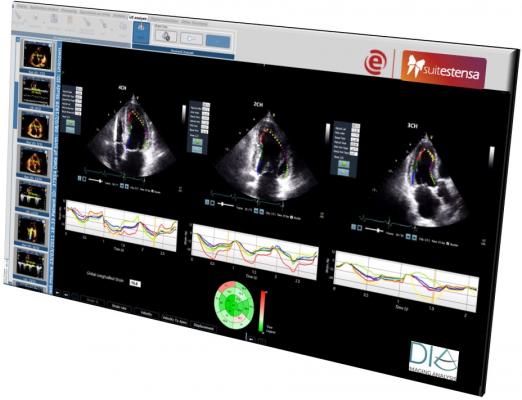
April 16, 2019 — DiA Imaging Analysis has partnered with the Italian healthcare IT company Ebit (Esaote Group), to offer DiA’s LVivo Cardiac Toolbox as an integrated part of Ebit's Suitestensa CVIS (cardiovascular information system). The LVivo Cardiac Toolbox is designed to analyze cardiac ultrasound images based on more objective and reproducible information, as opposed to manual measurement or visual analysis methods that are currently being used.
DiA’s LVivo Toolbox is based on advanced pattern recognition and machine learning algorithms that automatically imitate the way the human eye detects borders and motion. DiA's automated tools deliver fast and accurate clinical indications to support the decision-making process. LVivo is both vendor-neutral and easily implementable as part of the daily evaluation workflow. The integration of the LVivo toolbox will enable the support of DICOM clips from all ultrasound devices used at the lab, making LVivo AI analysis accessible to all lab users using the picture archiving and communication system (PACS) and ultimately improving patient care.
Ebit’s cardiovascular software Suitestensa is a vendor-neutral comprehensive enterprise platform that allows physicians to archive, manage and share data reports and clinical images produced by any cardiological equipment. Ebit will offer the LVivo Toolbox as an integrated part of its PACS viewer. The integration has been designed around the same human interface of Suitestensa CVIS in order to assure the best user experience and efficient workflow.
For more information: www.dia-analysis.com, www.esaote.com


 March 06, 2026
March 06, 2026 









