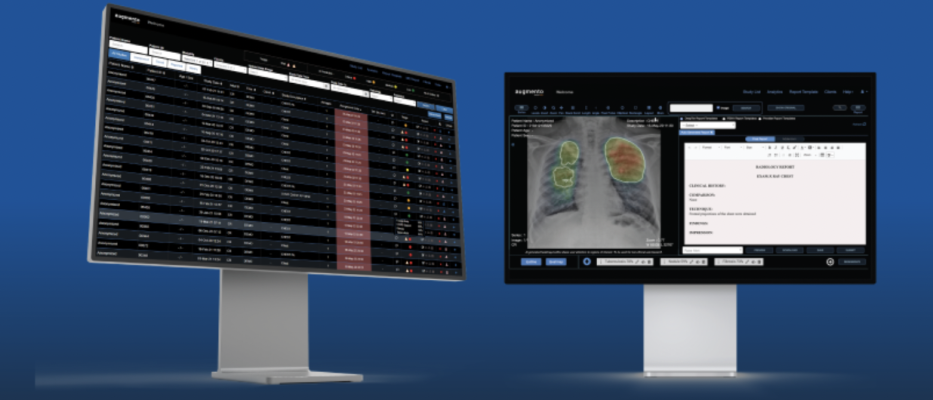
DeepTek.ai, A Pune, India-based radiology artificial intelligence (AI) company, has announced that its AI-powered Radiology Workflow Management Solution, Augmento, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for clinical use. Image courtesy: DeepTek.ai
May 4, 2023 — DeepTek.ai, A Pune, India-based radiology artificial intelligence (AI) company, has announced that its AI-powered Radiology Workflow Management Solution, Augmento, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for clinical use. In so doing, Augmento becomes India's first U.S. FDA-cleared AI-powered radiology workflow management solution.
According to the DeepTek.ai announcement, the Indian Radiological and Imaging Association has released a report indicating a critical shortage of radiologists in the country. The ratio of radiologists to population is only one per 100,000 people, which is significantly lower than the World Health Organization's recommended standard of one radiologist per 10,000 people, noted the report. This issue of radiologist shortage is not confined to developing countries such as India; even developed countries such as the UK, Japan, and Singapore are grappling with this problem, cited the statement, adding that the shortfall has a notable impact on the ability to diagnose patients accurately and in a timely manner, resulting in delayed treatments, higher expenses, and poor health outcomes.
“The U.S. FDA clearance is a big shot in our arm,” said DeepTek.ai Co-Founder and CEO of DeepTek.ai Dr. Amit Kharat. In a company statement announcing the clearance, Kharat added, “It enables us to take our offering to the U.S., which is the largest global healthcare market. Our vision is to revolutionize radiology with the power of AI and make it more affordable, accessible, and accurate.”
AI in healthcare is in a very early phase,” noted said Ashutosh Pathak, Chief Technology Officer of DeepTek.ai. “AI results are still opaque/black box in nature and hence doctors are not quite comfortable relying on them. ‘Responsible AI’ addresses these issues and brings techniques to enable building trust between the Doctors and the AI results. DeepTek has been working at the forefront of this technology,” Pathak added. The statement also reported that DeepTek.ai’s AI-Powered Augmento platform brings in AI in a seamless and responsible way to revolutionize radiology workflows and transform productivity, quality, and patient care.
Augmento is currently servicing 350+ hospitals and imaging centers across India touching about 60,000 lives every month. Outside India, it is deployed across several countries in the APAC region. The Singapore government as a part of its National AI strategy has been adopting the Augmento platform in key hospitals.
Incorporated in the U.S. in 2017, DeepTek.ai has successfully established commercial adoption of AI technology in clinical practice, creating clear and quantifiable value for patients, hospitals, and radiologists, reports the company, noting its offerings touch lives across India, Japan, Singapore, Thailand, Philippines, Nigeria, Kenya, and several other countries. It also reports that DeepTek.ai has a team of 200+ members with a unique mix of technology experts and radiologists.
For more information, visit www.deeptek.ai.
Related Coverage:


 March 06, 2026
March 06, 2026