April 9, 2019 — Digital pathology company Deep Lens Inc. announced the closing of a $14 million Series A financing that will further expand the company’s artificial intelligence (AI) and platform product development activities. Deep Lens is focused on AI-driven digital pathology for clinical trial recruitment at the time of diagnosis.
The financing was led by Northpond Ventures. Existing investors Rev1 Ventures, Sierra Ventures and Tamarind-Hill Partners also participated in this round, which comes just three months after Deep Lens closed its VC-led seed equity round. Funding to date now totals $17.5 million.
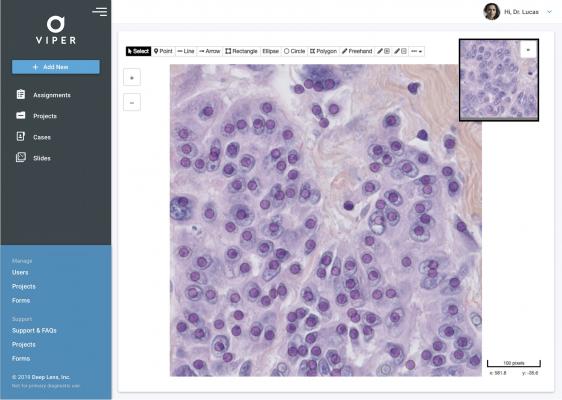
The company’s flagship technology, called VIPER (Virtual Imaging for Pathology Education and Research), was originally developed for research purposes in Columbus, Ohio. There it was the de-facto platform for global oncology studies including The Cancer Genome Atlas Project (TCGA) and others, before being commercialized by Deep Lens. VIPER combines AI with advanced pathology workflows, while also facilitating peer-to-peer collaboration and patient identification for clinical trials. The ultimate aim is to provide users with fast and accurate information, along with expert consultation, for better patient care and advanced clinical research.
Patient recruitment for clinical trials remains a time-intensive, costly barrier to the execution of drug development programs. More than 14,000 oncology clinical trials are actively recruiting patients1, yet estimates put the rate of participation as low as 3 percent of potential trial candidates.2 Deep Lens’ VIPER platform enables the pathologist in clinical trial recruitment. By identifying eligible patients at the time of their diagnosis, much sooner than current methods, VIPER can help fast-track trial enrollment and potentially shorten the duration of the trial.
For more information: www.deeplens.ai
References
1. https://clinicaltrials.gov, Last accessed February 28, 2019


 February 13, 2026
February 13, 2026 









